Off-the-shelf Lead mAbs, Ready to Licensing Out
Antibody drugs are one of the most revolutionary breakthroughs in biopharmaceuticals. With their high specificity, low toxicity, and broad therapeutic potential, they have become the fastest-growing segment of the global pharmaceutical market. However, antibody drug development is a costly and time-consuming undertaking, with the preclinical development phase alone taking at least three to five years and requiring millions, if not tens of millions, of dollars in investment.
To help pharmaceutical companies accelerate the preclinical development of antibody drugs, DIMA BIOTECH has launched the “All Druggable Targets” lead antibody molecule development program, leveraging its proprietary, innovative single B cell development platform. DIMA BIOTECH will generate lead antibody molecules and corresponding B cell seed libraries targeting all druggable targets. Clients can directly import lead antibody molecules from DIMA BIOTECH or rapidly screen for additional lead antibodies from the B cell seed library. Compared to traditional hybridoma technology, DIMA BIOTECH can save clients up to nearly a year in development time.
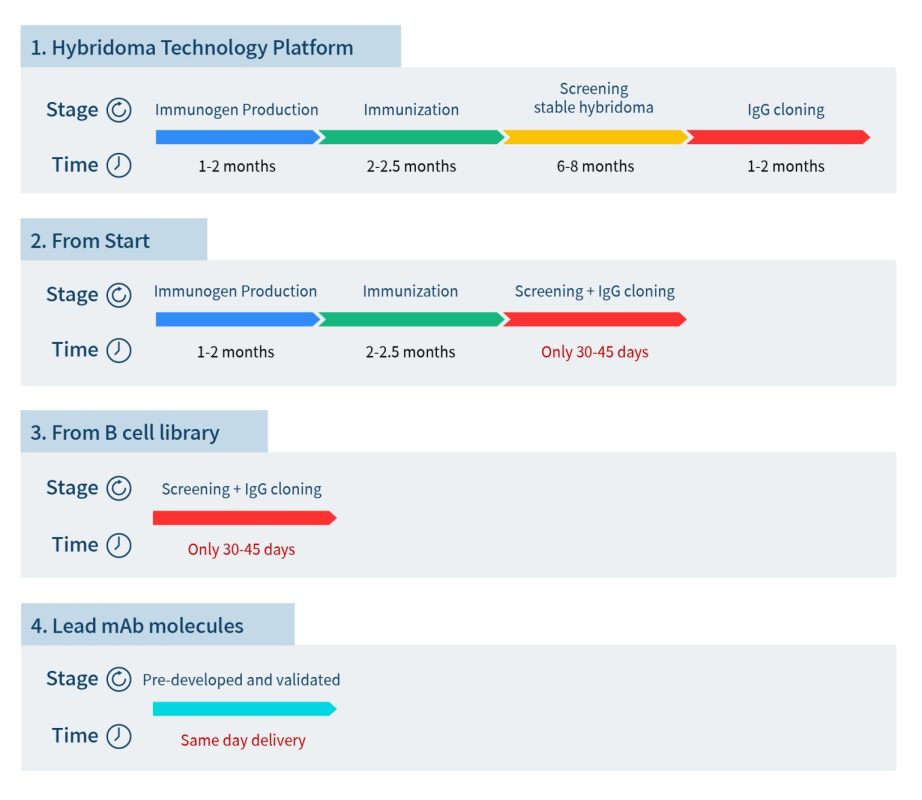
To date, DIMA BIOTECH has screened over 5,000 lead antibody sequences, covering over 500 druggable targets. All of these have been sequenced and are available for global licensing. For some popular target sequences, DIMA BIOTECH has also conducted human-monkey cross-talk and in vitro functional validation. Clients can immediately import sequences and data packages for functional validation, enabling them to be one step ahead and seize market opportunities.
DIMA's Features
Off-the-Shelf Lead mAbs

- No Upfront: Pay after testing
- No Waiting: Same-day delivery
- No risk: No payment if validation is invalid
- High quality: All antibodies are FC-validated
- High efficiency: Save at least 8 months of R&D time
B Cell Seed library
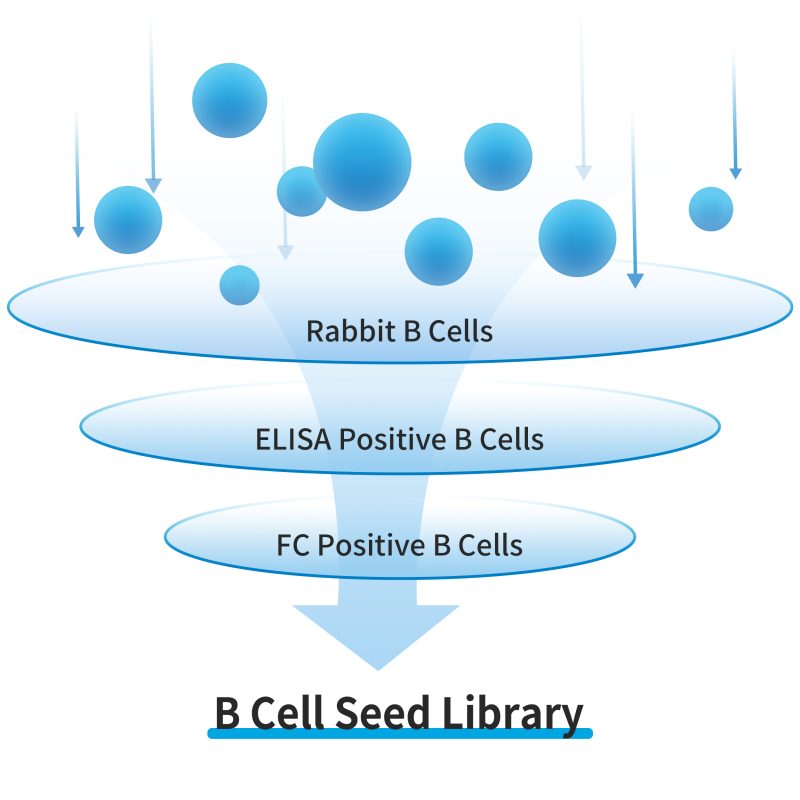
- No risk: No payment if validation is invalid
- Diversity: Multiple FACS binders
- High quality: Serum-free polyclonal Ab identification and FACS functional validation
- High efficiency: Save at least 6 months of R&D time
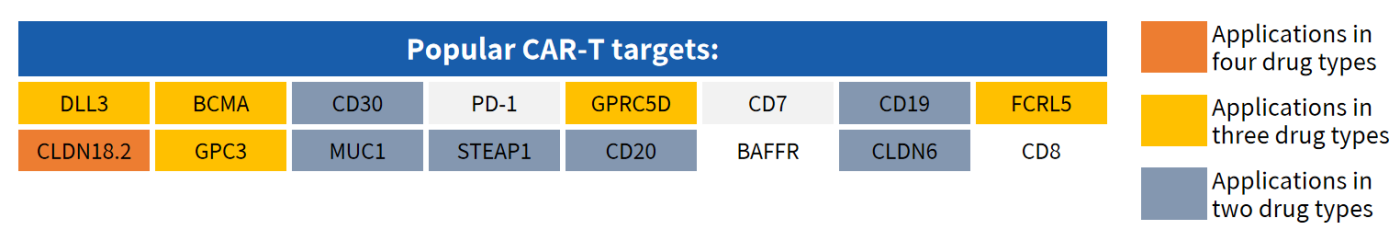
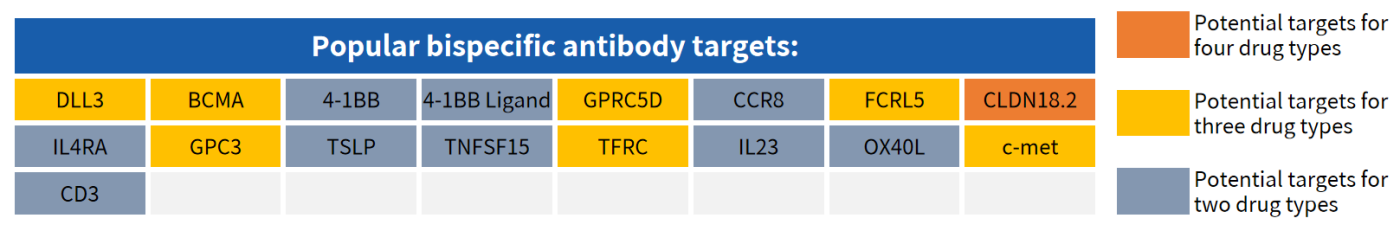
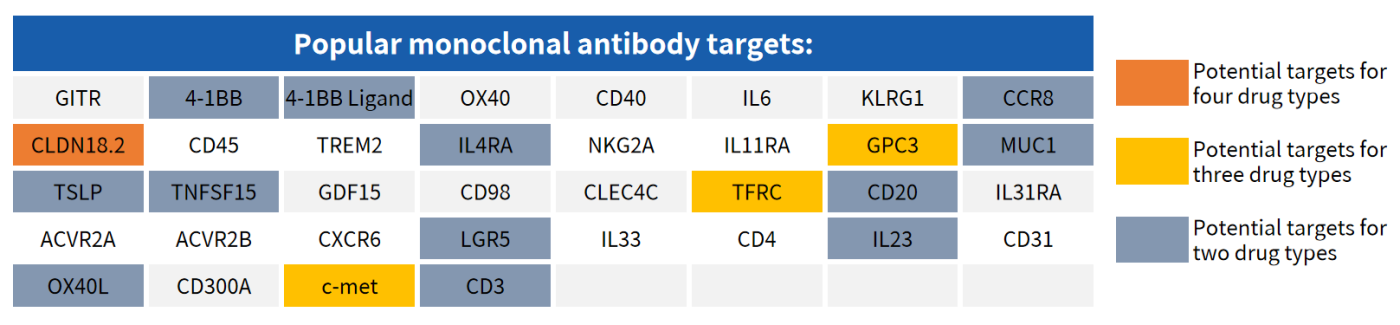
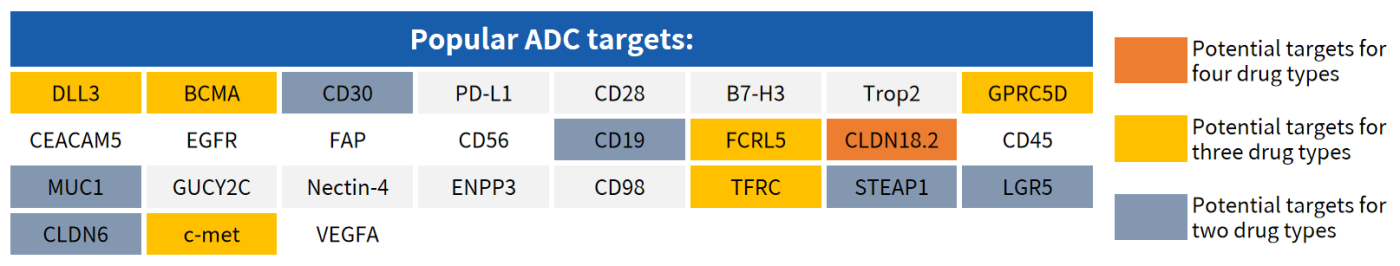
Partial Popular Targets
Case Study
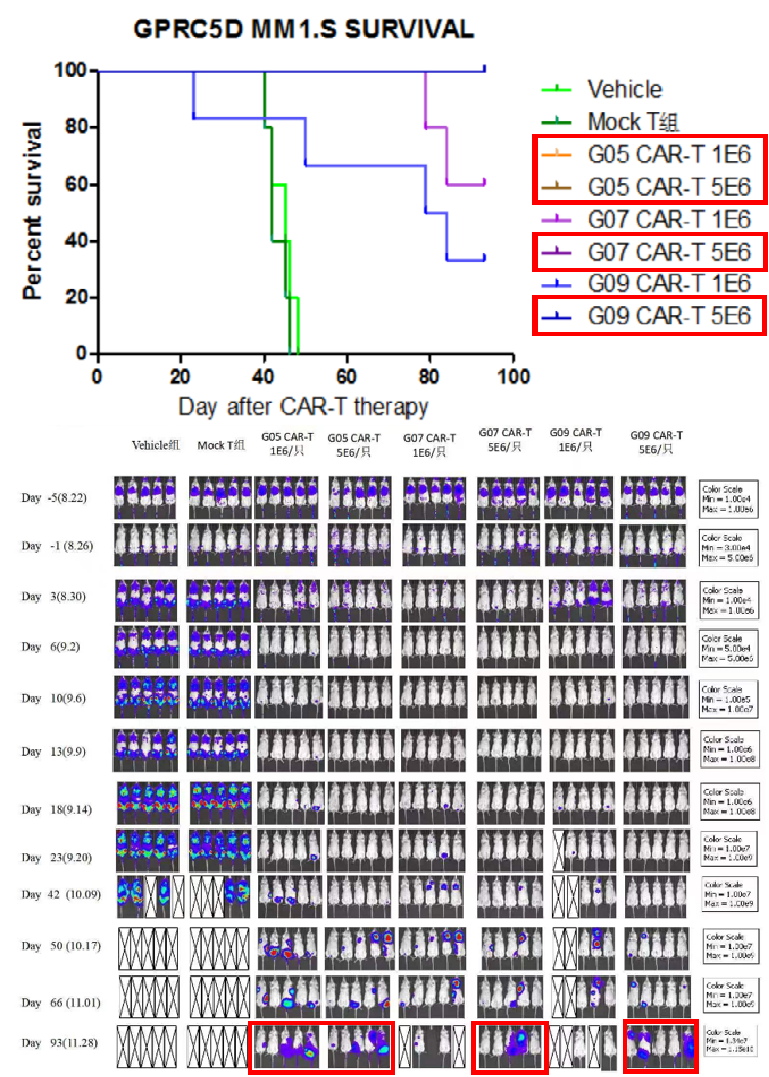
Animal survival data summary on selected CAR constructs
93 days post CAR T-cells infusion, red box-labelled groups of mice are still alive.
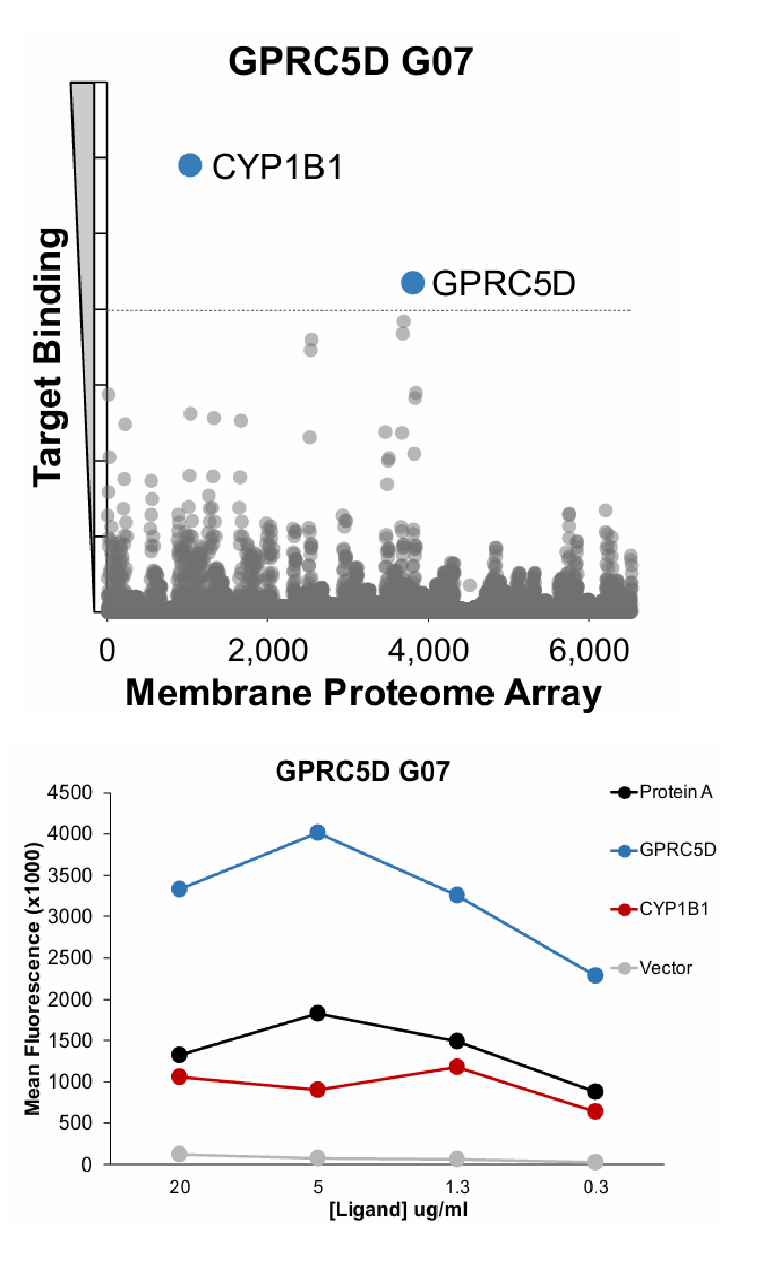
GPRC5D G07 membrane protein array (MPA)
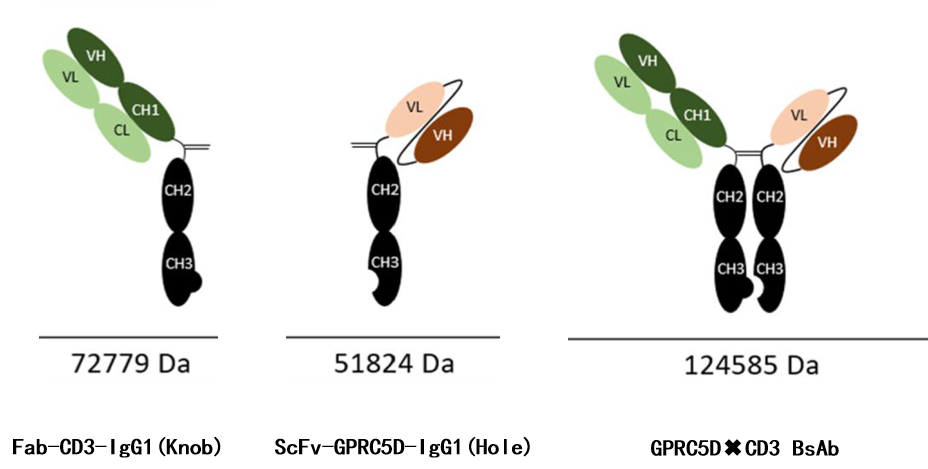
DIMA’s GPRC5D x CD3 BsAb format (KIH)
DIMA utilizes Fab-ScFV-IgG1(KIH) format CD3 :SP34
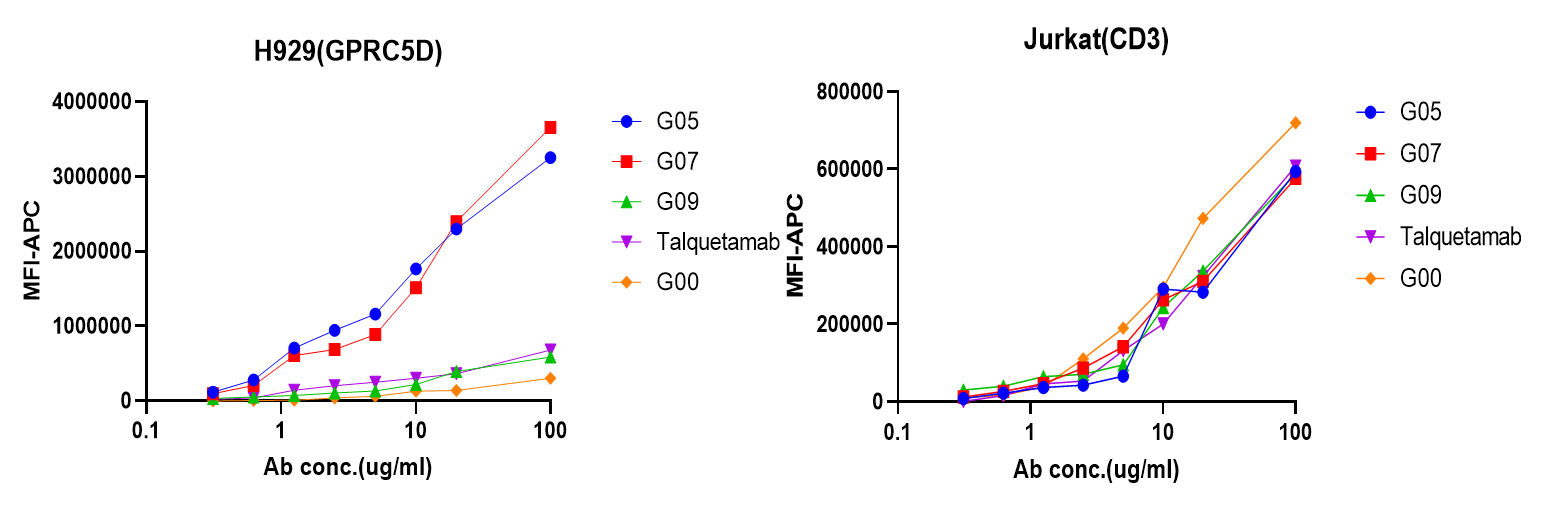
BsAb(GPRC5D X CD3) affinity ranking by flow assay
G05, 07 and 09 are DIMA’s BsAb; Talquetamab is J&J’s BsAb; G00 is the BsAb derived from scFv of Eureka and BMS’s MCARH109
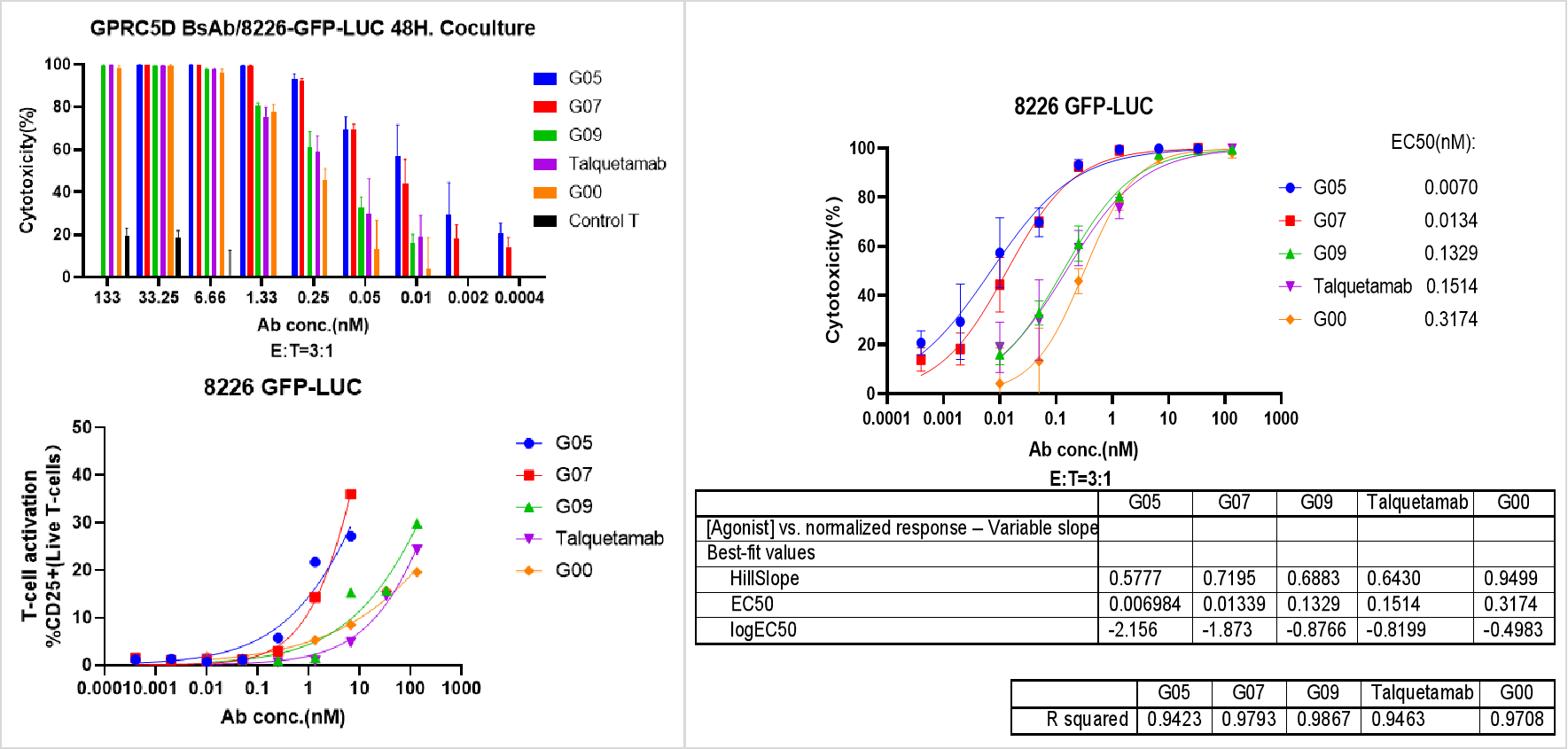
In Vitro tumor cell (8226-GFP-LUC) killing assays with BsAbs
T cells and tumor cells were mixed at 3:1 ratio and different concentration of BsAbs were added and further incubated for 48hrs before assay analysis.
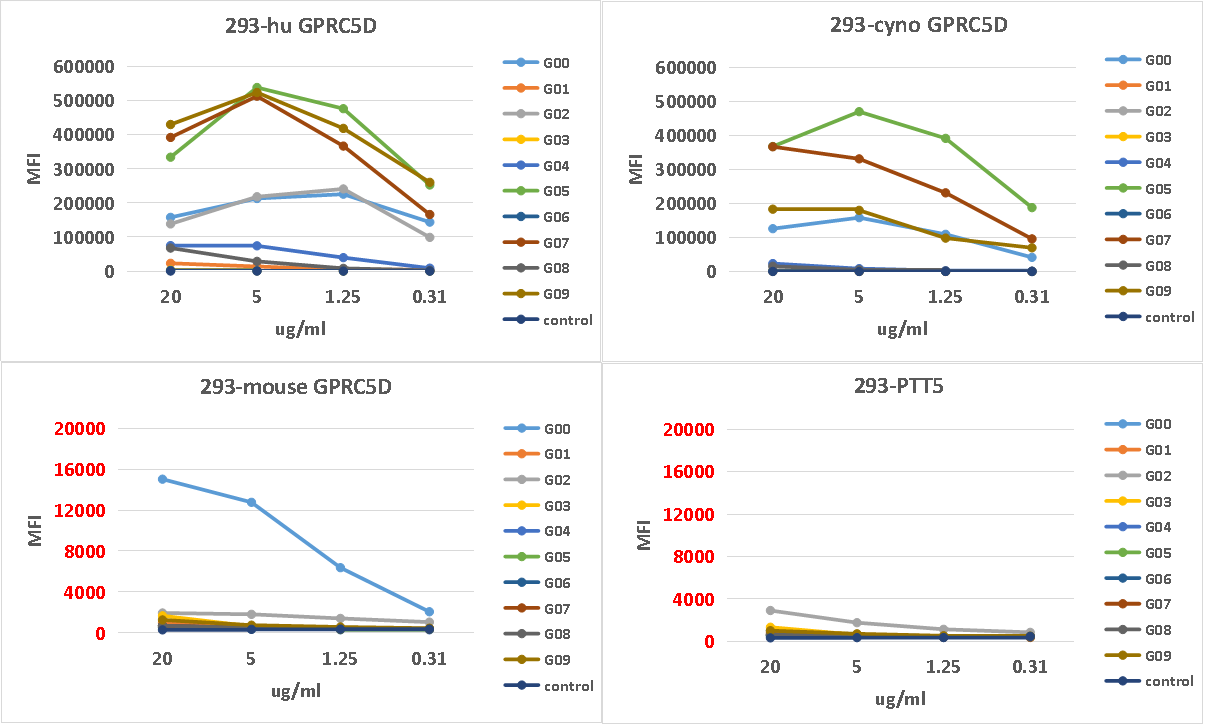
Cyno and mouse cross-reactivity tests
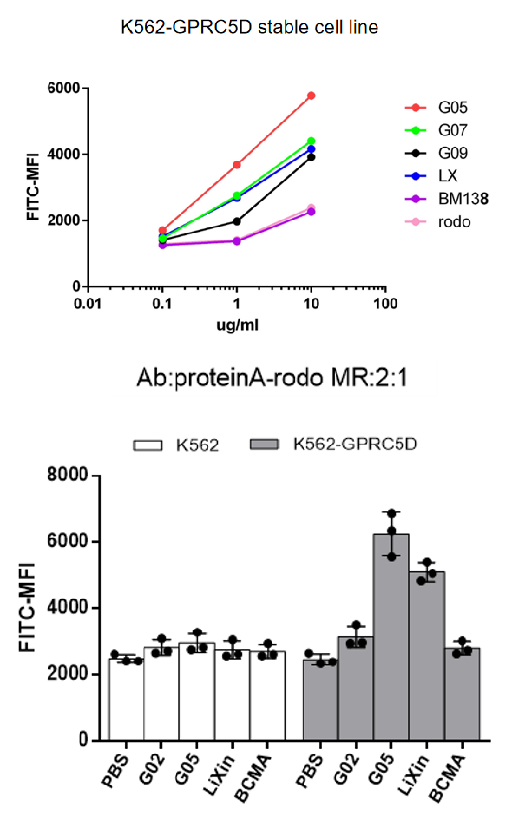
Clone G05 exhibits strong internalization property
Clone G05 exhibits strong internalization property