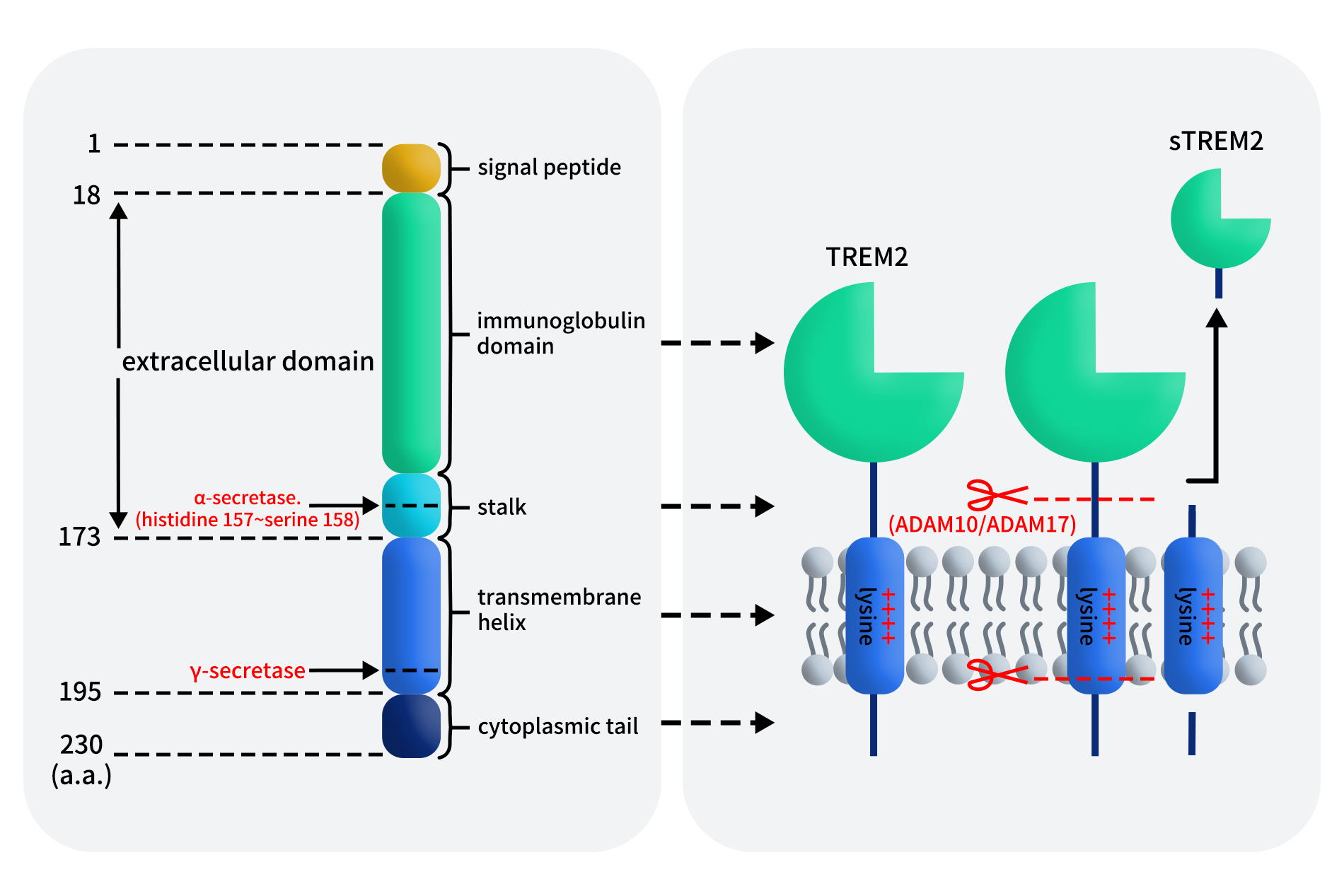
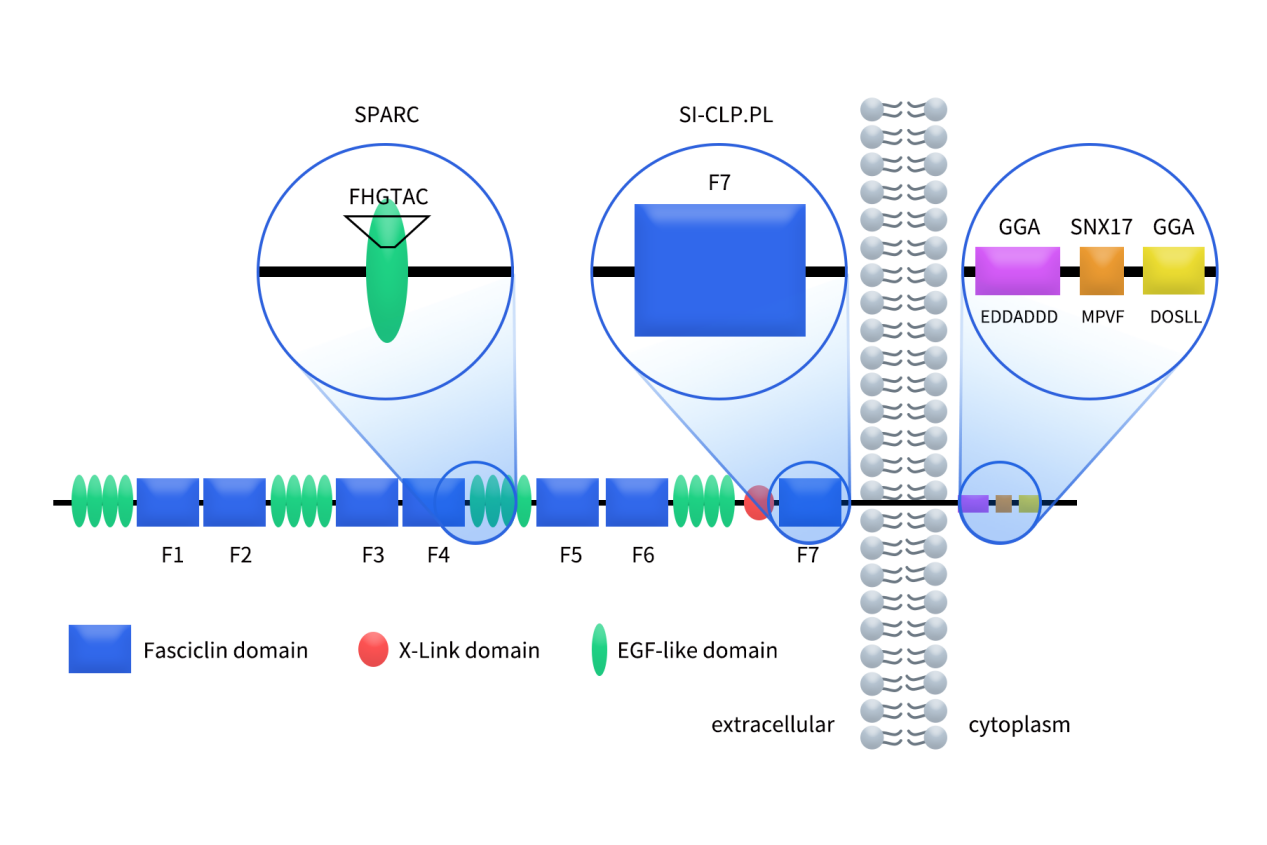
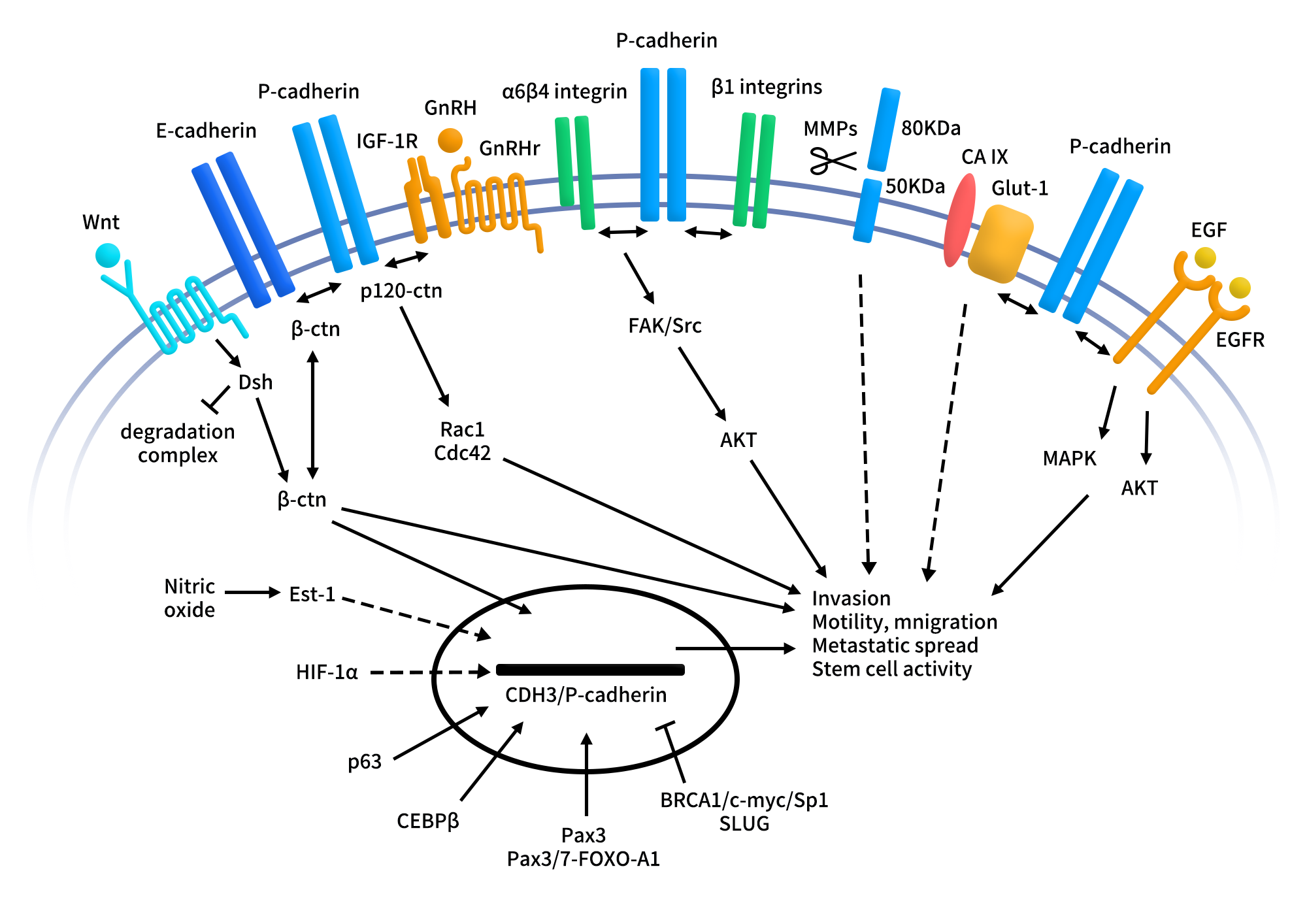
Between 2024 and 2025, Programmed Death-Ligand 1 (PD-L1) targeted therapies have once again become a focal point in global cancer immunotherapy. On one hand, PD-L1 based bispecific antibodies, such as PD-L1xVEGF and PD-L1xCTLA-4, have continued to release encouraging mid to late stage clinical data in lung cancer, hepatocellular carcinoma, and gastric cancer, demonstrating efficacy that may surpass traditional monoclonal antibodies. On the other hand, as first-generation PD-L1 monoclonal antibodies, including Atezolizumab, Durvalumab, and Avelumab, approach patent expiration, pharmaceutical companies worldwide are accelerating efforts toward differentiated innovation.
PD-L1 is no longer viewed merely as a standalone drug target. Instead, it has emerged as a central hub for immune combination therapies and multifunctional molecular designs, fundamentally reshaping the R&D landscape of PD-L1 targeted drugs.