On December 7, 2023, AbelZeta announced a collaboration with AstraZeneca to jointly develop C-CAR031, a chimeric antigen receptor T-cell (CAR-T) therapy targeting Glypican-3 (GPC3) for hepatocellular carcinoma (HCC). According to the agreement, AbelZeta is eligible for milestone payments and licensing fees for its global development. The drug will be exclusively developed, manufactured, and commercialized by AstraZeneca outside China. Why is GPC3 attracting the attention of pharmaceutical giant AstraZeneca? And what other pharmaceutical companies are focusing on immune therapies targeting GPC3?
1. What are the Structure and Expression Characteristics of GPC3 Protein
GPC3, also known as DGSX, GTR2-2, MXR7, OCI-5, is a member of the heparan sulfate (HS) proteoglycan family encoded by the GPC3 gene. The GPC3 gene is located on chromosome Xq26, and its encoded protein consists of a core protein with 580 amino acids and two HS chains located at the C-terminus. The GPC3 core protein is anchored to the cell membrane surface through glycosylphosphatidylinositol (GPI), and it can be cleaved into approximately 40 KDa N-terminal soluble protein (sGPC3) and 30 KDa C-terminal membrane protein. The cleaved sGPC3 can be released from cancer cells into circulation (Figure 1).
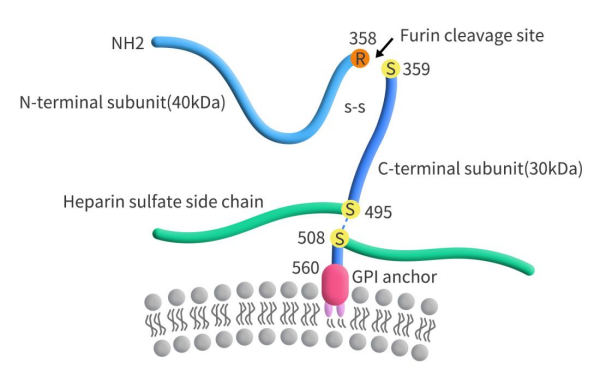
Figure 1. The structure of GPC3 [1]
GPC3 is widely expressed in human embryos and plays a crucial role in morphogenesis and growth through mechanisms involving insulin-like growth factors, bone morphogenetic proteins (BMP), fibroblast growth factors (FGF), or hedgehog proteins (Hh) signaling pathways [2] [3]. GPC3 can be detected in the fetal liver of embryos between 18 and 30 weeks of gestation, but the expression of GPC3 protein is rarely observed in any normal adult liver tissue, including conditions such as fatty liver, cirrhosis, hepatitis, or liver damage [4] [5]. This expression pattern was first reported by Hsu et al. in a study in 1997, indicating that the mRNA and protein levels of GPC3 in most HCC are higher compared to normal liver tissue, cholangiocarcinoma, and liver metastases [6]. This expression characteristic has positioned GPC3 as an ideal diagnostic and therapeutic target for HCC.
2. GPC3 and HCC
Apart from serving as a diagnostic marker for HCC, GPC3 also plays a crucial role in the progression of HCC. Research indicates that GPC3 can participate in and promote the growth and invasion of HCC by binding to Wnt and FGF signal proteins or growth factors through its HS side chains. The exact mechanism of GPC3 protein in hepatocellular carcinoma is not fully understood. Some studies suggest that GPC3 functions through the classical Wnt/β-catenin signaling pathway, activating the Wnt signaling pathway to upregulate Wnt genes, thereby promoting tumor growth.
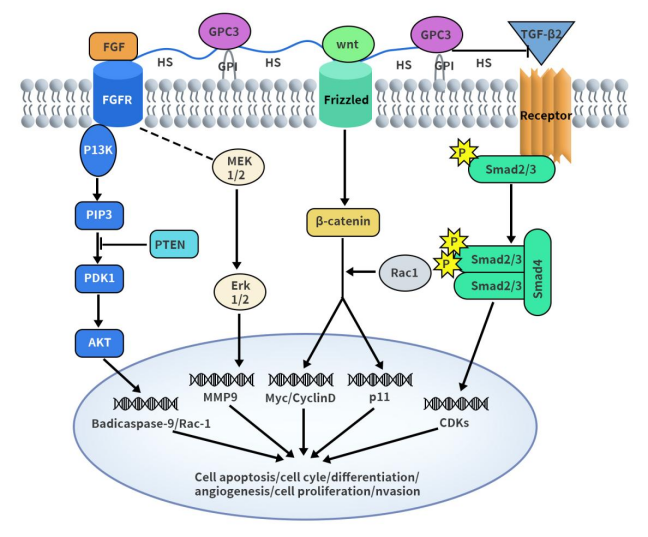
Figure 2. The diagram of possible GPC3-mediated signaling pathway in HCC progression [7]
3. Therapeutic Strategies Targeting GPC3
The research journey for GPC3-targeted drugs has faced challenges. Clinical outcomes of single GPC3 monoclonal antibodies have been unsatisfactory, showing no significant differences in overall survival and progression-free survival compared to control groups. Currently, drug development targeting GPC3 in clinical trials primarily focuses on Phase I, involving antibody drugs and cell therapies, with CAR-T therapy having the highest number of clinical trial cases.
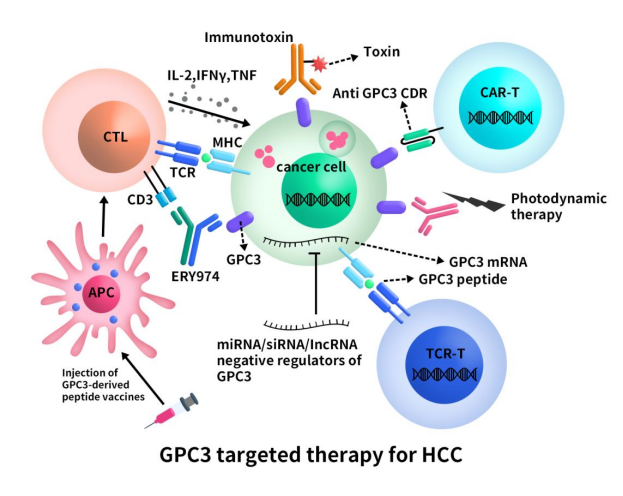
Figure 3. the diagram of GPC3 targeted therapy for HCC [8]
3.1 Antibody Drugs
- Codrituzumab (Monoclonal Antibody)
Codrituzumab, also known as GC33 or RO5137382, is a humanized monoclonal antibody targeting GPC3 developed by Chugai Pharmaceutical in collaboration with Roche. GC33 is the first humanized recombinant monoclonal antibody developed against GPC3. Results from a Phase II clinical trial (NCT01507168) published in 2016 showed that Codrituzumab alone did not demonstrate satisfactory clinical efficacy in second-line HCC patients. In June 2021, Chugai Pharmaceutical restarted a Phase I trial for Codrituzumab (NCT04928677), aiming to further explore its clinical value in solid tumors. The trial is recruiting.
- ERY974 (Bispecific Antibody)
ERY974, also known as ERY101EG, is a GPC3/CD3 bispecific antibody derived from GPC3 monoclonal antibody by Chugai Pharmaceutical. ERY974 can recognize both GPC3 and CD3 antigens, forming an immune synapse between T cells and tumor cells, mediating T cell killing of GPC3-overexpressing tumor cells. Currently, a new Phase I clinical study for ERY974 (NCT05022927) is underway to evaluate the safety, tolerability, and preliminary efficacy of ERY974 in combination with Tocilicumab (IL-6R), Atezolizumab (PD-L1), and Bevacizumab (VEGF-A) in advanced hepatocellular carcinoma patients. In September 2022, Sano, Y et al. published results in Nature Communications, showing that the combination of ERY974 with chemotherapy can more effectively enhance the efficacy of ERY974 in non-inflammatory tumors, further validating the development direction of ERY974’s combination therapy.
- CM350 (Bispecific Antibody)
CM350 is a GPC3xCD3 bispecific antibody developed by Keymed Biosciences based on their proprietary nTCE bispecific antibody platform. CM350 is currently in phase I clinical trial for the treatment of solid tumors that express GPC3, such as liver cancer, lung cancer, gastric cancer and esophageal cancer. CM350 activates T cells by simultaneously targeting GPC3 on tumor cells and CD3 on T cells, redirecting T cells to target tumor cells for elimination.
3.2 CAR-T Therapy
- BOXR1030
BOXR1030, developed by SOTIO Biotech based on the proprietary BOXR™ platform, is designed for the treatment of various solid tumors expressing GPC3. The structure of BOXR1030 is shown in the figure below. It is a second-generation CAR with a humanized GPC3-targeting scFv, 4-1BB co-stimulatory domain, and CD3ζ signaling domain. Utilizing the P2A sequence to link the codon-optimized GOT2, the impact of T cell metabolism on CAR-T cell function is evaluated. CAR-T cells are then generated through γ-retroviral transduction. Preclinical studies indicate that compared to traditional GPC3 CAR-T cells, BOXR1030 T cells exhibit improved proliferation under hypoxic and low glucose conditions, as well as enhanced tumor-killing capabilities. Moreover, in tumor-infiltrating lymphocytes isolated from animals, BOXR1030 shows greater resistance to functional impairment and fewer markers of exhaustion compared to control cells. The initial indications for BOXR1030 include hepatocellular carcinoma, squamous cell lung carcinoma, and mucinous/round cell liposarcoma. Currently, it has entered clinical trials and is undergoing the Phase 1/2 DUET-01 clinical trial (NCT05120271), which is in recruiting.

Figure 4. The structure of BOXR1030
- CT011 & CT0180 & CT0181
CT011, CT0180 and CT0181 are GPC3 antibody drugs developed by CARsgen Therapeutics and differ in the design of CAR structure.
CT011 is an autologous GPC3 CAR-T candidate for the treatment of HCC. In 2019, it received IND approval from the National Medical Products Administration (NMPA) for the treatment of GPC3-positive solid tumor patients, marking China’s first CAR-T cell therapy IND for solid tumors. CARsgen Therapeutics completed the patient enrollment for the Phase I clinical trial in China (NCT03884751). On January 15, 2024, the China NMPA approved the IND application for using CT011 to treat GPC3-positive stage IIIa HCC patients at risk of recurrence after surgical resection.
CT0180 is an autologous T-cell product expressing a fusion protein targeting GPC3 antibody and T-cell receptor. Preclinical studies demonstrated that CT0180 effectively recognizes and kills GPC3-positive liver cancer cells in a mouse xenograft model, significantly inhibiting HCC tumor growth. It is currently in an open-label, dose-escalation Phase I clinical trial (NCT04756648) to evaluate the safety, preliminary efficacy, and pharmacokinetics of CT0180 in late-stage HCC patients with GPC3 expression. The trial is in recruiting.
CT0181 is an autologous T-cell product expressing a fusion protein targeting GPC3 antibody and T-cell receptor, along with co-expression of IL-7 cytokine. Preclinical studies indicated that compared to GPC3 CAR-T cells, CT0181 demonstrated superior anti-tumor efficacy, T-cell persistence, and immunologic memory in solid tumor xenografts with low cytokine release. A Phase I clinical trial (NCT04973098) has been initiated in China to evaluate the safety and efficacy of CT0181 in treating hepatocellular carcinoma.
- B010-A
B010-A, Developed by Tongji University and Shanghai Pharmaceuticals, is GPC3-targeted CAR-T therapy with the addition of the SPH-Engine structure for enhanced CAR-T killing activity and intracellular circulation in late-stage solid tumors. In preclinical studies, B010-A has shown superior in vivo and in vitro anti-tumor activity and improved safety compared to traditional CAR-T therapies. Currently, an early-phase I clinical trial (NCT05070156) has been initiated in China. This open-label, single-arm study aims to observe the safety and tolerability of B010-A in treating late-stage HCC.
- TAK-102
TAK-102 (also known as NIB102), jointly developed by Takeda Pharmaceutical and Noile-Immune Biotech, is an experimental therapy that uses genetically modified T cells to target GPC3. It is designed to enhance the survival and function of the T cells by expressing two additional genes: interleukin-7 (IL-7) and chemokine ligand 19 (CCL19). These genes are expected to help the T cells expand, persist, and migrate to the tumor site. It is based on the PRIME technology developed by Noile-Immune Biotech. TAK-102 is one of the novel approaches to improve the efficacy and safety of CAR T cell therapy for solid tumors. Mid-term clinical data presented at SITC-2022 showed that, as of March 25, 2022, among four patients treated with TAK-102, there were no dose-limiting toxicities, cytokine release syndrome, or neurotoxicity. Two patients achieved stable disease (SD) accompanied by changes in tumor markers such as AFP and LDH. TAK-102 exhibited good pharmacokinetic behavior at lower dose levels, with evidence of dose-dependent effects. Dose escalation studies are still ongoing (NCT04405778).
- Ori-C101
Ori-C101, developed by Oricell Therapeutics, is a GPC3-targeted chimeric antigen receptor (CAR) T-cell therapy with a unique signaling activation component (Ori) for enhanced expansion efficiency of memory immune cells, strengthening CAR-T cell anti-tumor activity and persistence.
Clinical data presented at the 2022 ASCO showed that Ori-C101 demonstrated excellent safety and efficacy in GPC3-positive late-stage liver cancer patients. The objective response rate (ORR) was 44%, and the disease control rate (DCR) was 78%. The longest follow-up period exceeded 22 months, with ongoing follow-up. A Phase I clinical trial (NCT05652920) is currently ongoing to evaluate the safety, pharmacokinetics, and preliminary efficacy of Ori-C101 in treating late-stage hepatocellular.
- TC-CAR-031
TC-CAR-031 (C-CAR031), developed by AstraZeneca, is an Autologous anti-GPC3 armored CAR-T therapy designed for hepatocellular carcinoma HCC. As Figure 5 shows, it consists of three components, GPC3 CAR, T2A peptide, and armored Dominant Negative Transforming Growth Factor-βII Receptor (TGFβRIIDN). GPC3 CAR contains the intracellular domain expressing GPC3-targeting antibody, 4-1BB, and CD3z fusion protein. T2A peptide allows equimolar expression of two transgenic products. Expression of TGFβRIIDN protects cells against immune-suppressive tumor microenvironments.

Figure 5. The structure of C-CAR031
On December 7th, AbelZeta announced a joint development agreement with AstraZeneca for C-CAR031. At the 2023 AACR Annual Meeting, AbelZeta disclosed a researcher-initiated clinical trial (NCT05155189) conducted in China. This marks the first human trial for C-CAR031, aiming to investigate its feasibility, safety, and preliminary efficacy in treating HCC patients. The study is currently in the enrollment phase. Preliminary data showcased the safety, efficacy, pharmacokinetics (PK), and pharmacodynamics (PD) of C-CAR031. The data indicates that C-CAR031 exhibits promising anti-tumor activity and robust PK/PD characteristics in patients with advanced liver cell carcinoma.
- More GPC3 CAR-T Therapies
| Drug Name | Company | Phase | Indication | NCT Number |
| JWATM214 | JW Therapeutics | N/A | HCC | NCT05926726 NCT06144385 |
| ECT204 | Eureka Therapeutics | Phase I | HCC | NCT04864054 |
| CG1003 | Carsgen | Phase I | Lung squamous cell carcinoma | NCT02876978 NCT02395250 |
| GB5011 | GENE | Phase I/II | HCC | NCT02715362 NCT03130712 |
| GLYCAR T cell therapy | Baylor College of Medicine | Phase I | HCC | NCT04377932 |
| Anti-GPC3 CAR T-cell therapy | NanjingUniversity | Phase I | HCC | NCT04121273 |
| Anti-GPC3 chimeric antigen receptor T cell therapy | Hunan ZhaotaiYongren GuangdongZhaotai In VivoBiotechnology | Phase I | HCC | NCT03198546 |
4. DIMA’s GPC3 CAR-T Cell Preparation and Testing
DIMA has its own independently developed CAR-T lead molecule screening experimental platform. Over the past two years, we have completed the development of antibody lead molecules for over 200 projects, and some CAR-T lead molecules have reached the pre-clinical experimental stage. Specifically targeting the GPC3 target, DIMA Biotech has successfully prepared CAR-T cells for multiple antibody molecules and conducted a 24-hour short-term cytotoxicity test. Feel free to inquire about our testing services.
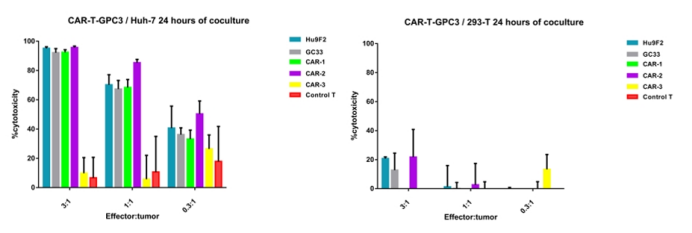
Figure 6. The data of CAR-T-GPC3/Huh-7 (Left) or 293-T (Right) 24 hours of co-culture
In addition, to support the development of GPC3-targeted biopharmaceuticals, DIMA Biotech has also developed a complete series of products targeting the GPC3 target. The products include functionally active proteins, flow-validated antibodies, and reference antibodies. Our services encompass antibody humanization and affinity maturation. Furthermore, to expedite GPC3-targeted drug development, DIMA Biotech has established a B-cell seed library for the GPC3 target, allowing the acquisition of GPC3 antibody lead molecules in as little as 20 days.
| Product types | Name | Cat. | Validation |
| Recombinant Protein | Human GPC3 Protein, hFc Tag | PME100113 | SDS-PAGE, ELISA |
| mAb | Anti-GPC3 antibody(DMC371); IgG1 Chimeric mAb | DMC100371 | FC |
| Benchmark | Anti-GPC3(Hu9F2) mAb | BME100147 | ELISA |
| Anti-GPC3(codrituzumab biosimilar) mAb | BME100083 | ELISA, FC |
Reference:
[1] Ho, Mitchell, and Hungnam Kim. “Glypican-3: a new target for cancer immunotherapy.” European journal of cancer 47.3 (2011): 333- 338.
[2] Filmus J, Capurro M, Rast J. Glypicans. Genome Biol. 2008;9(5):224.
[3] Traister A, Shi W, Filmus J. Mammalian Notum induces the release of glypicans and other GPI anchored proteins from the cell surface. Biochem J. 2008;410(3):503–511.
[4] Yamauchi N, Watanabe A, Hishinuma M, et al. The glypican 3 oncofetal protein is a promising diagnostic marker for hepatocellular carcinoma. Mod Pathol. 2005;18(12):1591–1598.
[5] Zhang L, Liu H, Sun L, Li N, Ding H, Zheng J. Glypican-3 as a potential differential diagnosis marker for hepatocellular carcinoma: a tissue microarray-based study. Acta Histochem. 2012;114(6):547–552.
[6] Hsu HC, Cheng W, Lai PL. Cloning and expression of a developmentally regulated transcript MXR7 in hepatocellular carcinoma: biological significance and temporospatial distribution. Cancer Res. 1997;57(22):5179–5184.
[7] Wu Y, Liu H, Ding H. GPC-3 in hepatocellular carcinoma: current perspectives. J Hepatocell Carcinoma. 2016 Nov 8;3:63-67.
[8] Zheng, Xiufeng et al. “Glypican-3: A Novel and Promising Target for the Treatment of Hepatocellular Carcinoma.” Front Oncol. 2022 Feb 16.
[9] Sano, Y., Azuma, Y., Tsunenari, T. et al. Combination of T cell-redirecting bispecific antibody ERY974 and chemotherapy reciprocally enhances efficacy against non-inflamed tumours. Nat Commun 13, 5265 (2022).
