CCR1 (C-C motif chemokine receptor 1) is a G protein-coupled receptor (GPCR) involved in inflammatory responses and immune cell migration. In recent years, with the deepening research on immune-related diseases and the tumor microenvironment, CCR1 has gradually emerged as an important therapeutic target for autoimmune diseases, tumors, and organ fibrosis.
1. Overview of CCR1
Chemokine monomers consist of a central three-stranded β-sheet structure overlaid with a C-terminal α-helix, and contain an N-terminal region that is structurally unstable yet crucial for receptor activation. Chemokines are highly homologous to each other, with approximately 20%–50% similarity in their gene and amino acid sequences. Based on the arrangement of cysteine residues at their N-terminus, chemokines can be divided into four subfamilies: CXC, CC, C, and CX3C. Among them, CC and CXC are the two main subfamilies. In CC chemokines, the first two cysteine residues are adjacent, whereas in CXC chemokines, a single amino acid residue separates the first two cysteines. C chemokines lack the first and third cysteines, while in CX3C chemokines, three amino acid residues are inserted between the first two cysteines.
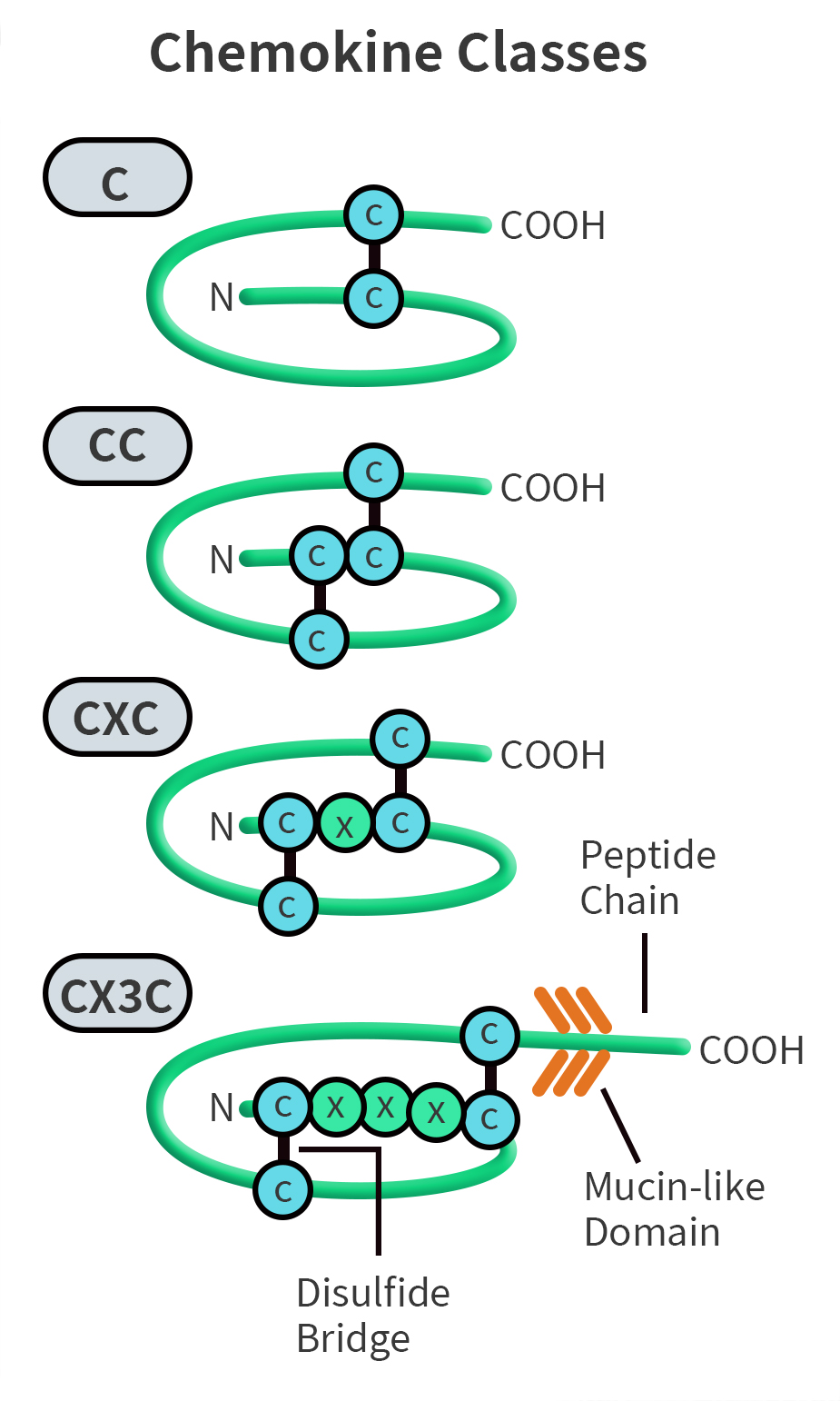
Fig 1.CC Chemokine Family Classification.[1]
The CC chemokine receptors (CCRs) are important members of the G protein-coupled receptor (GPCR) family, characterized by seven transmembrane α-helical domains. This transmembrane structure divides the receptor into an intracellular C-terminal region, three intracellular loops, three extracellular loops, and an extracellular N-terminus. Upon binding to one of the intracellular loops, G proteins initiate intracellular signaling cascades that regulate a variety of biological functions. Chemokine receptors are composed of approximately 340 to 370 amino acids, with sequence homology ranging from 25% to 80%. Based on the types of chemokine ligands they bind, chemokine receptors are classified into four subfamilies: CXCR, CCR, XCR, and CX3CR.
CCR1 is a member of the CC chemokine receptor family and primarily binds to ligands such as CCL3 (MIP-1α), CCL5 (RANTES), CCL7, and CCL23, thereby inducing immune cell migration and activation. As a typical GPCR, CCR1 features the hallmark seven-transmembrane domain structure.
Key structural features of CCR1 include:
- Extracellular N-terminal domain: Responsible for ligand binding
- Transmembrane helical region: The seven transmembrane α-helices form the core of signal activation
- Intracellular loops (ICLs): Regulate interactions with G proteins and arrestins
- Intracellular C-terminal domain: Involved in receptor internalization and signal termination
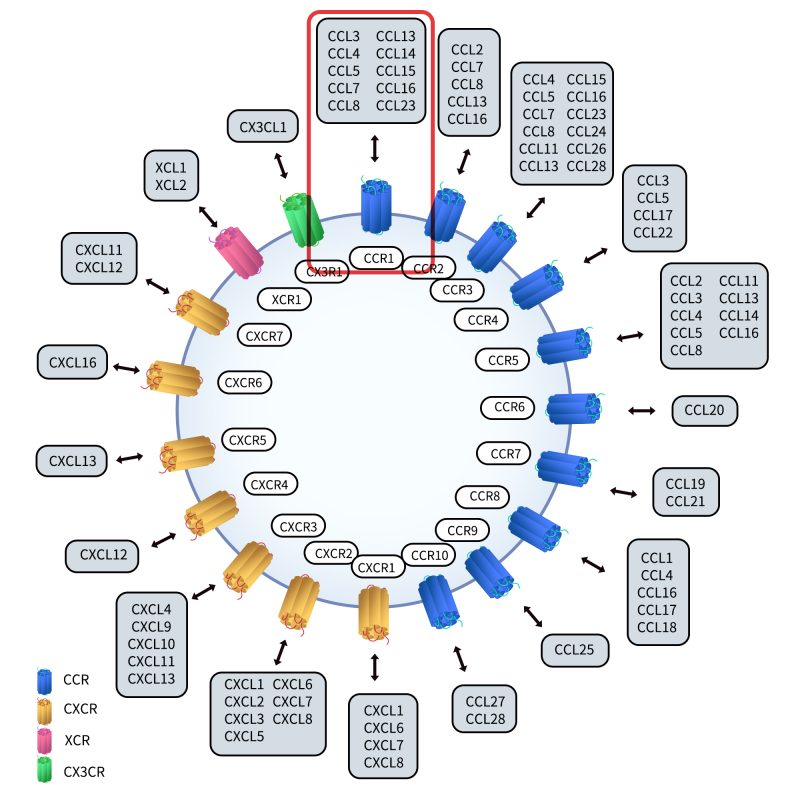
Fig 2.CCR Classification.[2]
Currently, there is no high-resolution crystal structure available for CCR1. However, homology modeling suggests that it shares a high degree of structural similarity with other CC chemokine receptors, such as CCR5.
2. CCR1 Expression and Signaling Pathways
2.1 CCR1 Expression
CCR1 is primarily expressed on the cell membranes of various immune cells, including monocytes, immature dendritic cells, T lymphocytes, basophils, eosinophils, neutrophils, natural killer (NK) cells, mast cells, endothelial cells, human umbilical cord blood (HUCB) cells, and others. It mainly mediates chemokine signal transduction. In addition, some studies have confirmed that CCR1 is also expressed in non-hematopoietic cells within the central nervous system (CNS). Previous research has shown that CCR1 can be expressed in various brain cell types, including microvascular endothelial cells, vascular smooth muscle cells, microglia, astrocytes, and neurons.
Its main functions include:
① Mediating immune cell chemotaxis: guiding immune cells to sites of inflammation in response to inflammatory signals;
② Regulating cell activation and the release of inflammatory cytokines;
③ Promoting antigen presentation and the formation of immune memory;
④ Acting as a precise “GPS” for immune surveillance and stress responses, playing a key role in maintaining immune homeostasis.
2.2 CCR1-Mediated Signaling Pathways
CCR1 mainly binds to ligands such as CCL3 and CCL5, mediating the migration of immune cells (e.g., monocytes, macrophages, and T cells) to sites of inflammation. Upon activation, CCR1 signals through Gi proteins to reduce intracellular cAMP levels and activate the PI3K/Akt and PLC/Ca2+/PKC pathways. Additionally, it can engage β-arrestin to trigger the MAPK pathway, thereby regulating cell chemotaxis, adhesion, activation, and inflammatory responses. CCR1 signaling plays a critical role in various inflammatory conditions, autoimmune diseases, and within the tumor microenvironment.
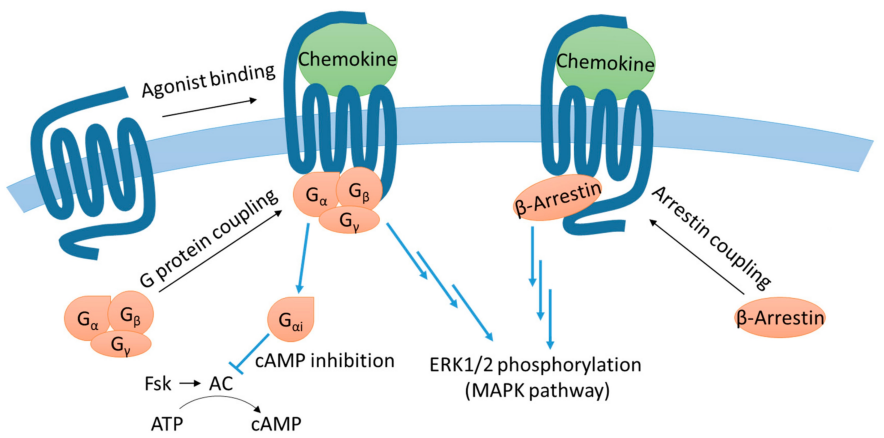
Fig 3.Simplified diagram of CCR1 signaling pathway.[3]
3. CCR1-Related Diseases
Overactivation or aberrant expression of CCR1 is closely associated with a variety of diseases:
3.1 Autoimmune and Inflammatory Diseases
Rheumatoid Arthritis (RA): CCR1⁺monocytes and macrophages can migrate into synovial tissue, triggering inflammation and bone destruction.
Multiple Sclerosis (MS): CCR1 regulates immune cell infiltration in the central nervous system, contributing to demyelinating lesions.
Inflammatory Bowel Disease (IBD): Elevated CCR1 expression is associated with abnormal activation of immune cells in the intestinal mucosa.
3.2 Tumor Immunity and Metastasis
In various solid tumors (such as breast and prostate cancer), CCR1 promotes the recruitment of tumor-associated macrophages (TAMs), facilitating immune evasion and tumor invasion/metastasis.
Certain cancer cells also express CCR1, increasing their responsiveness to chemokines and enhancing metastatic potential.
3.3 Organ Fibrosis
In pulmonary fibrosis and glomerulonephritis, CCR1 regulates the migration and activation of inflammatory cells and fibroblasts, serving as a “trigger” for tissue fibrosis.
4. Drug Development Targeting CCR1
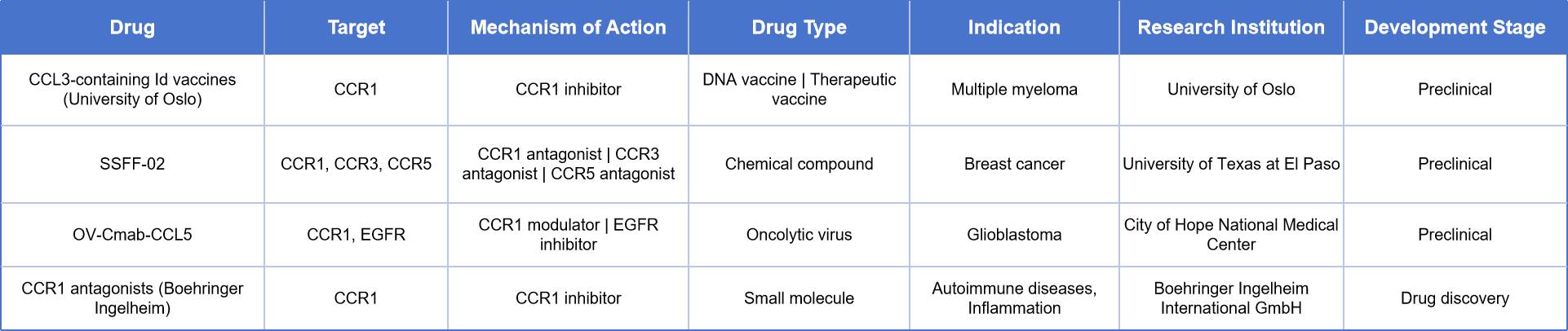
Although no CCR1-targeted therapies have been approved yet, several drug candidates are currently in clinical development, mainly for inflammatory, autoimmune, and cancer-related indications. Four drugs are in development, spanning various modalities including DNA vaccines, small-molecule drugs, antibodies, and oncolytic viruses:
CCL3-containing Id Vaccine developed by the University of Oslo (Norway): Acts via CCR1 antagonism for the treatment of multiple myeloma; currently in preclinical stages.
SSFF-02 from the University of Texas at El Paso: A small-molecule drug targeting CCR1/3/5, offering a new therapeutic option for breast cancer.
OV-Cmab-CCL5 from City of Hope Medical Center: An oncolytic virus therapy targeting both CCR1 and EGFR, being developed for glioblastoma.
CCR1 Antagonists from Boehringer Ingelheim: A series of small-molecule inhibitors aimed at treating autoimmune and inflammatory diseases; currently in the discovery phase.
An additional 16 drug candidates targeting CCR1 are either inactive or have been discontinued. Among them, several CCR1 antagonists had advanced to Phase I or II clinical trials, with indications primarily including inflammatory conditions, rheumatoid arthritis, and multiple sclerosis.
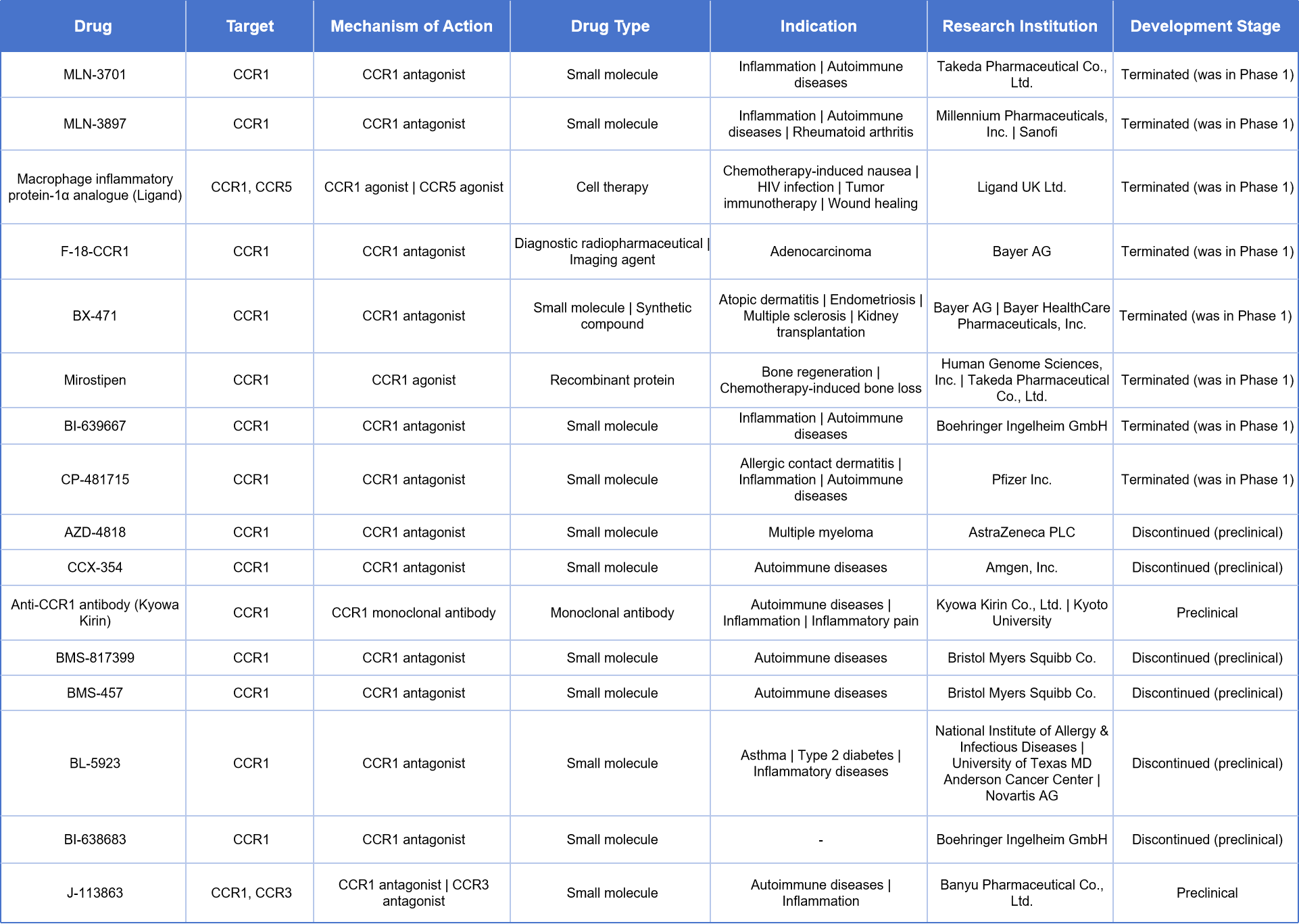
- Takeda’s MLN-3701 and Millennium/Sanofi’s MLN-3897 are both small-molecule CCR1 antagonists developed for inflammatory diseases, multiple sclerosis, and rheumatoid arthritis. Both programs have been terminated during Phase I clinical trials.
- F-18-CCR1 developed by Bayer AG is a radiolabeled diagnostic ligand explored for imaging in Alzheimer’s disease.
- BX-471, also developed by Bayer, was intended for autoimmune conditions such as psoriasis and transplant-related rejection. It has also been discontinued after Phase I trials.
- Mirostipen, a CCR1 agonist co-developed by Human Genome Sciences and Takeda, was originally intended for bone marrow suppression and hematopoietic support, but has also been terminated.
Several other projects remain in clinical development but have shown no recent progress:
- Anti-CCR1 antibody by Kyowa Kirin is a monoclonal antibody targeting CCR1 for inflammatory and autoimmune diseases, currently in the discovery/early research stage.
- AZD-4818 (AstraZeneca), CCX-354 (Amgen), BMS-457, and BMS-817399 are small-molecule CCR1 antagonists being developed for rheumatoid arthritis and chronic inflammation. These candidates are currently in preclinical or early-stage development.
In addition to single-target drugs, there are also two multifunctional agents targeting multiple receptors (CCR1, CCR3, CCR5):
- Macrophage inflammatory protein-1α analogue, developed by Ligand, is a chemokine-based biologic that acts on both CCR1 and CCR5, being explored for applications such as HIV infection and chemotherapy-induced tissue damage.
- J-113863 (Banyu) is a dual antagonist targeting CCR1 and CCR3, under investigation for multiple sclerosis and currently in the preclinical phase.
To date, no CCR1-targeting drugs have been approved for market. The challenges in CCR1 drug development include:
Receptor redundancy: Functional overlap between CCR1 and other chemokine receptors such as CCR3 and CCR5 limits the effectiveness of single-target interventions.
Balancing selectivity and safety: It is crucial to avoid impairing normal immune defense functions.
Lack of structural data for target validation: The absence of high-resolution CCR1 structures hampers structure-based drug design (SBDD).
CCR1 serves as a critical link between inflammation, immunity, and cancer. Despite challenges such as receptor redundancy and selectivity, it continues to show great therapeutic potential in areas such as rheumatoid arthritis, cancer immunotherapy, and organ fibrosis.
5. DIMA Biotech CCR1-Related Products
DIMA Biotech offers a wide range of in-stock CCR1-related products, including recombinant proteins, full-length membrane proteins, recombinant monoclonal antibodies, biotin/PE-labeled antibodies, and CDX tissue slides. We also provide comprehensive services such as protein/antibody customization, antibody humanization, affinity maturation, and generation of stable cell lines. Additionally, we have established a B cell seed library specific for the CCR1 target, enabling the screening of lead antibody candidates within just 20 days.
- Proteins & Antibodies
| Type | SKU | Product Name |
| ECD Proteins | PME100921 | Human CCR1 Protein, hFc Tag |
| Full Length Transmembrane Proteins | FLP120094 | Human CCR1-Strep full length protein-synthetic nanodisc |
| FLP100094 | Human CCR1 full length protein-synthetic nanodisc | |
| Monoclonal Antibodies | DMC100465 | Anti-CCR1 antibody(DMC465); IgG1 Chimeric mAb |
| Biotinylated mAb | DMC100465B | Biotinylated Anti-CCR1 antibody(DMC465); IgG1 Chimeric mAb |
| PE-conjugated mAb | DMC100465P | PE-conjugated Anti-CCR1 antibody(DMC465); IgG1 Chimeric mAb |
- CCR1 Lead Molecule Research Progress

References:
- Markl, F., et al., Utilizing chemokines in cancer immunotherapy. Trends Cancer, 2022. 8(8): p. 670-682.
- Vilgelm, A.E. and A. Richmond, Chemokines Modulate Immune Surveillance in Tumorigenesis, Metastasis, and Response to Immunotherapy. Front Immunol, 2019. 10: p. 333.
- Sanchez, J., et al., Influence of Chemokine N-Terminal Modification on Biased Agonism at the Chemokine Receptor CCR1. Int J Mol Sci, 2019. 20(10).
- Gilchrist, A. and S.L. Echeverria, Targeting Chemokine Receptor CCR1 as a Potential Therapeutic Approach for Multiple Myeloma. Front Endocrinol (Lausanne), 2022. 13: p. 846310.
- Tian, Q., et al., Inflammatory Role of CCR1 in the Central Nervous System. Neuroimmunomodulation, 2024. 31(1): p. 173-182.
