When it comes to DLL3, people immediately think of Rova-T, the ADC drug targeting the DLL3 receptor that dashed AbbVie’s $5.8 billion expectations. AbbVie’s setback had once caused a decline in the enthusiasm for DLL3-targeted treatments. However, the spotlight returned when Amgen unveiled the DLL3/CD3 bispecific antibody Tarlatamab data at the 2023 ESMO conference. On December 13 of the same year, Amgen announced that the BLA application for Tarlatamab had been accepted by the FDA and granted priority review status. With this announcement, the momentum for DLL3-targeted therapies continued to surge. But what exactly is DLL3? And which pharmaceutical companies are currently advancing therapeutic pipelines targeting DLL3?
1. Structure and Distribution of DLL3
Delta-Like Ligand 3 (DLL3) is a member of the Delta-like ligands (DLLs) family. DLLs are single-pass transmembrane proteins attached to the cell surface, and besides DLL3, this family includes two other members, DLL1 and DLL4. The genes encoding DLL1, DLL3, and DLL4 are located on chromosomes 6q27, 19q13.2, and 15q15.1, respectively [1].
The human DLL3 protein comprises 619 amino acids, characterized by a conserved N-terminal DSL (Delta, Serrate, Lag2) domain consisting of 40 amino acids, six EGF-like repeat sequences, and a transmembrane domain. Notably, the SDL domain, crucial for binding with Notch receptors, is highly conserved within the ligand family. As illustrated in Figure 1, DLL1 and DLL4 differ from DLL3 as they possess eight EGF-like repeat sequences. Additionally, DLL1 and DLL4 feature a PDZ-binding motif at their C-terminus, a structural element absent in DLL3 [2]. Throughout evolution, DLL3 has demonstrated a high degree of conservation, with human and mouse DLL3 protein sequences exhibiting homology of up to 82%.
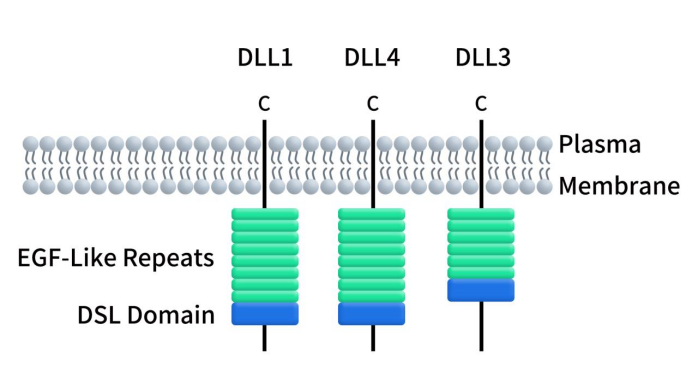
Figure 1. The structure of DLLs [1]
DLL3 RNA is predominantly expressed in the brain, endocrine tissues, and blood, with minimal expression in normal tissues outside these regions. Studies reveal that DLL3 is highly expressed in approximately 80% of Small Cell Lung Cancer (SCLC) cases and other neuroendocrine tumors. The differential expression of DLL3 in normal and tumor cells makes it an attractive target for tumor-selective therapeutic interventions. The heightened expression of DLL3 in SCLC and other neuroendocrine tumors underscores its potential as a key player in these malignancies, presenting an opportunity for targeted therapeutic strategies.
2.DLL3 and the Notch Signaling Pathway
DLL3 acts as an inhibitory ligand in the Notch signaling pathway [3], playing a crucial role in Notch signal transduction. The Notch pathway is a highly conserved intercellular signaling pathway that regulates various processes in growth and development, including the differentiation of pluripotent progenitor cells, cell apoptosis, cell proliferation, and the formation of cell boundaries.
Typically, the Notch signaling pathway regulates cell fate through local cell-to-cell interactions. In mammals, there are four Notch receptors (Notch1, Notch2, Notch3, Notch4) and five Notch ligands (DLL1, DLL3, DLL4, Jagged 1–2). Notch receptors undergo furin protease cleavage (S1 cleavage) in the Golgi apparatus, leading to the formation of transmembrane heterodimers transported to the cell surface. Upon interaction with Notch ligands on adjacent cells, the Notch receptor undergoes two successive cleavages. First, ADAM10/17 catalyzes peptide bond cleavage near the extracellular membrane (S2 cleavage), followed by cleavage by the γ-secretase complex (S3 cleavage) near the intracellular membrane, releasing the Notch intracellular domain (NICD). The released NICD enters the cell nucleus and forms a NICD/CSL transcriptional activation complex by binding to the DNA-binding protein CSL (CBF-1). This complex recruits MAML (Mastermind-like protein) and activates the transcription of Notch-targeted genes, including the Hes, Hey, HERP, and other transcription factor families [4] [5].
Prior to transportation to the cell membrane, DLLs undergo O-fucosylation in the Golgi apparatus to modulate DLL-Notch signaling. Upon binding to EGF on the cell membrane, DLLs activate the Notch signal through endocytosis. Post-endocytosis, DLLs are either degraded by proteases or lysosomes or recycled to the cell membrane surface. The degradation or recycling of endocytosed DLLs is controlled by the ubiquitination of the DLL intracellular domain (DICD). Apart from activating the Notch signal, DLLs can also inhibit the Notch signal through various pathways.
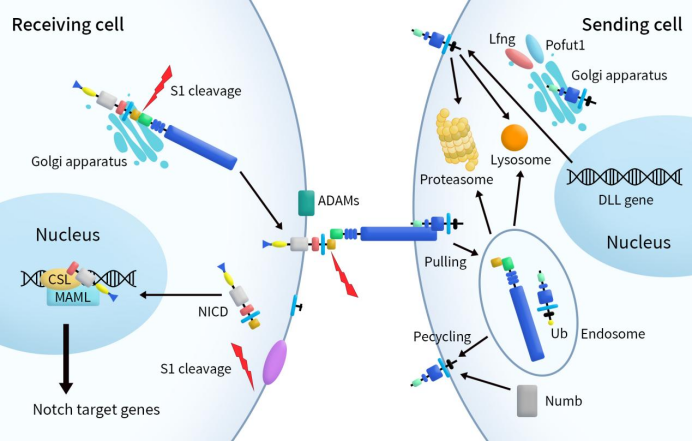
Figure 2. Notch ligands and Notch signaling [6]
Contrary to other mammalian Notch family members, DLL3 exhibits a unique localization during normal developmental processes. DLL3 is positioned within the Golgi apparatus and cytoplasmic vesicles. It interacts with unprocessed full-length Notch1 and DLL1, preventing their localization on the cell surface, thereby exerting inhibitory effects on the Notch signaling pathway. This distinctive characteristic of DLL3 in hindering the proper surface placement of Notch receptors contributes to its crucial role in negatively modulating the Notch signaling cascade during regular developmental processes.
3. DLL3 and SCLC
SCLC is a highly invasive, poorly differentiated neuroendocrine tumor and represents the most aggressive subtype of lung cancer. The progression of SCLC is closely associated with the Notch signaling pathway, a pathway that regulates fundamental processes essential for normal development. Inactivation mutations in this pathway can induce non-neuroendocrine tumor cells or tumor precursor cells to undergo neuroendocrine differentiation. DLL3, being the sole inhibitory ligand in the Notch signaling pathway, is highly expressed in 85% of SCLC cases but remains absent in normal lung tissues. This suggests a potential association between DLL3 and the occurrence of neuroendocrine tumors, although the exact mechanisms are not yet fully understood.
Furthermore, DLL3 serves as a downstream target of achaete-scute homolog 1 (ASCL1), playing a crucial role in neuroendocrine cell differentiation and SCLC growth. Research conducted by Furuta M [7] and others demonstrated that overexpression of DLL3 promotes the growth, migration, and invasion capabilities of SCLC cells. Similarly, Deng et al. found that in Lewis Lung Carcinoma (LLC) cells in mice, DLL3 enhances tumor growth in vivo by promoting Akt protein phosphorylation, inhibiting Notch receptor activity, reducing cell apoptosis, and increasing cell proliferation [8].
Beyond SCLC, DLL3 exhibits significantly upregulated expression and abnormal cell surface expression in Large Cell Neuroendocrine Lung Cancer (LCNEC), certain other neuroendocrine cancers in different locations, and prostate cancer. This distinctive ectopic expression makes DLL3 a potential therapeutic target in a variety of neuroendocrine malignancies.
4. Clinical Research Advances in DLL3-Targeted Therapies
Currently, there are a total of 16 therapies targeting DLL3, with 15 in various clinical stages and 1 in the preclinical stage. These therapeutic approaches encompass diverse types, including Antibody-Drug Conjugates (ADCs), Chimeric Antigen Receptor T-cell therapy (CAR-T), and antibody drugs.
- 4.1 DLL3-ADC
Rova-T, short for Rovalpituzumab tesirine, is an Antibody-Drug Conjugate (ADC) targeting DLL3. Initially developed by Stemcentrx, AbbVie acquired Stemcentrx for an initial payment of $5.8 billion in 2016, taking over the clinical development of Rova-T. The latest update indicates the termination of its development. Rova-T consists of a DLL3-specific humanized monoclonal antibody conjugated to a cleavable dipeptide linker and the pyrrolobenzodiazepine (PBD) dimer toxin.
Rova-T triggers receptor-mediated endocytosis by binding to DLL3 on the cell surface. The endocytic process internalizes the Rova-T-DLL3 complex into the cell, followed by fusion with lysosomes. In the presence of lysosomal proteases, the valine-alanine linker of Rova-T is cleaved, releasing PBD into the cytoplasm. Subsequently, the cytoplasmic PBD enters the nucleus, intercalates into DNA in a site-specific manner, induces DNA damage, and ultimately leads to cell death through apoptosis [9].
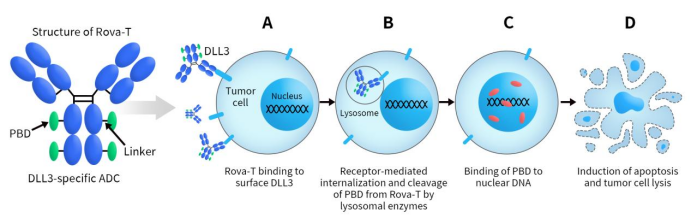
Figure 3. Mechanism of action of Rova-T
Rova-T underwent at least 10 clinical trials, including two Phase 3 trials. In 2016, the initial human studies of Rova-T in second- and third-line treatment for SCLC patients resulted in a 18% Objective Response Rate (ORR) and a 36% one-year survival rate. This outcome brought significant excitement to the industry, contributing to the sustained enthusiasm for DLL3 as a therapeutic target. Building on these promising results, AbbVie opted to skip Phase II clinical trials and initiated Phase III studies directly.
Unfortunately, both Phase III trials were prematurely terminated due to the failure to meet the pre-specified mid-term Progression-Free Survival (PFS) and/or Overall Survival (OS) primary endpoints. Despite the initial optimism, the discontinuation of Rova-T’s development by AbbVie was announced, taking into consideration the comprehensive analysis of results and other relevant factors. This highlights the challenges in translating early positive clinical signals into successful late-stage outcomes in the complex landscape of cancer therapeutics.
FZ-AD005, developed by Fudan Zhangjiang using its Linker-Drug platform (BB05 platform), represents the third new-generation ADC in their pipeline. This ADC product is created by coupling a recombinant human-mouse chimeric anti-DLL3 monoclonal antibody with a topoisomerase I inhibitor through the BB05 linker. In a significant milestone, the drug received clinical approval on December 22, 2023. The intended application of FZ-AD005 is for the treatment of advanced solid tumors, including but not limited to SCLC, Large Cell Neuroendocrine Lung Cancer, and prostate cancer.
YL212, also known as ZL-1310, is an ADC product targeting DLL3 developed by MediLink Therapeutics, featuring proprietary TAMLIN® technology. The TAMLIN® platform represents a novel ADC platform that leverages the tumor microenvironment to overcome challenges faced by current ADC drugs. Unlike traditional intracellular cleavage mechanisms, YL212 employs a dual mechanism involving extracellular and intracellular cleavage, making it a candidate molecule based on innovative ADC technology.
On April 27, 2023, MediLink Therapeutics and Remegen entered into a strategic cooperation and global licensing agreement for YL212. Subsequently, on December 1, 2023, China’s Center for Drug Evaluation (CDE) officially displayed the acceptance of Remegen’s clinical application for injectable ZL-1310. The clinical trial is currently in Phase I, focusing on cancer indications.
- 4.2 DLL3 Bi-specific Antibody
AMG 757, also known as Tarlatamab, is a bispecific antibody developed by Amgen that targets DLL3 and CD3. In December 2023, the Biologics License Application (BLA) for Tarlatamab was accepted by the FDA with priority review status. It is indicated for second-line SCLC and holds the potential to become the world’s first CD3/DLL3 bispecific antibody. Tarlatamab is a bispecific T-cell engager (TCE) with dual affinity for DLL3 on tumor cells and CD3 on T cells. This dual binding facilitates close interaction between tumor cells and autologous T cells, triggering the formation of an immune synapse and T-cell activation.
The characteristic features of this interaction include CD3 clustering, T-cell proliferation, and the release of perforin and granzyme. This series of events ultimately leads to tumor cell apoptosis and the amplification of T-cell responses. The Tarlatamab molecule is composed of two single-chain variable fragments (scFv) and includes a stable, inert Fc domain to enhance its half-life. Currently, six ongoing clinical studies are assessing the efficacy and safety of Tarlatamab.
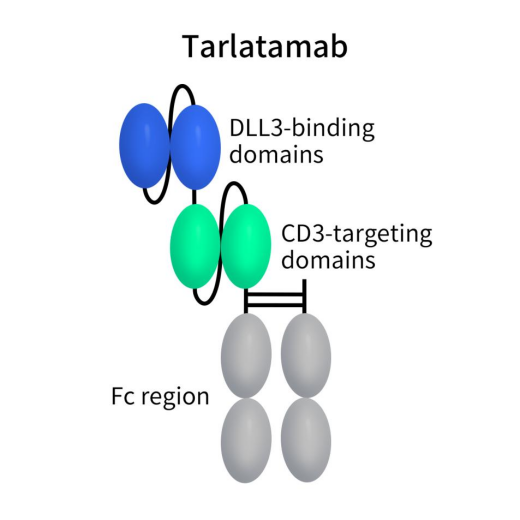
Figure 4. the structure of action of Tarlatamab
QLS31904, developed by Qilu Pharmaceutical, is a TCE bispecific antibody targeting DLL3/CD3. This drug comprises a specific Fab fragment targeting DLL3, an scFv fragment targeting CD3, and a modified Fc region to support heterodimerization. Currently in Phase I clinical trials, QLS31904 is intended for the treatment of advanced solid tumors, including SCLC.
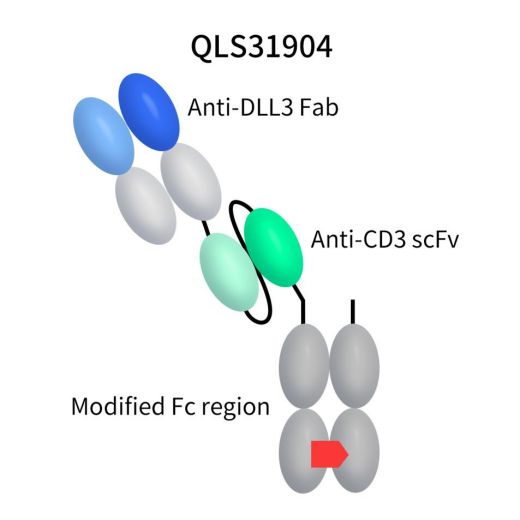
Figure 5. the structure of action of QLS31904
BI764532, developed by Boehringer Ingelheim, is another TCE bispecific antibody with an IgG framework targeting DLL3/CD3. In October 2023, BI 764532 received Fast Track designation from the FDA for the treatment of extensive-stage SCLC and advanced or metastatic extrapulmonary Neuroendocrine Cancer (NEC). Currently, BI764532 is in Phase II clinical trials.
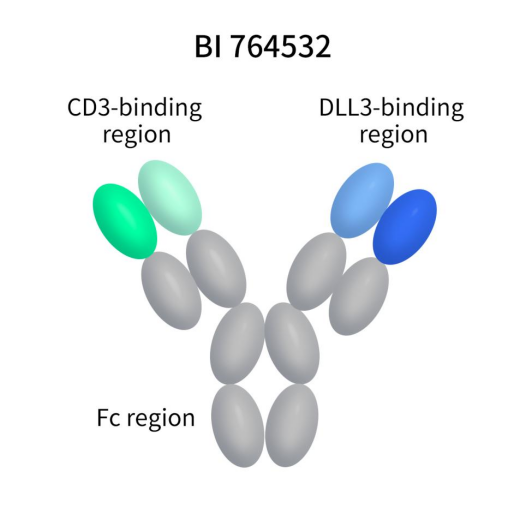
Figure 6. the structure of action of BI764532
PT-217, independently developed by Phanes Therapeutics, is a bispecific antibody targeting DLL3 and CD47, featuring a natural IgG structure. PT-217 is designed to directly eliminate tumor cells through the Antibody-Dependent Cellular Phagocytosis (ADCP) activity of macrophages and the Antibody-Dependent Cellular Cytotoxicity (ADCC) activity of Natural Killer (NK) cells. By simultaneously targeting the overexpressed DLL3 and CD47 on the surface of tumor cells, PT-217 aims to broaden the scope of tumor cell killing.
Moreover, PT-217 has the potential to induce the presentation of tumor neoantigens by guiding tumor cells into Antigen-Presenting Cells (APCs). Through the recognition of tumor neoantigens, it may indirectly activate T cells to target tumor cells with low or no expression of DLL3, stimulating the adaptive immune system. The anti-CD47 arm of PT-217 is highly differentiated, showing strong binding activity to CD47 on tumor cells while minimizing binding to human red blood cells in preclinical models. In 2022, PT-217 received approval from the U.S. Food and Drug Administration (FDA) to conduct a multicenter Phase I clinical trial in the United States. Additionally, it obtained orphan drug designation from the FDA in 2022 for the treatment of SCLC.
- 4.3 DLL3 Tri-specific antibody
HPN328, developed by Harpoon Therapeutics using its proprietary Tri-specific T cell Activating Construct (TriTAC®) platform, is a trispecific antibody comprising three humanized antibody-derived domains. These include an N-terminal domain binding to DLL3 on tumor cells, a middle domain binding to human serum albumin for half-life extension, and a C-terminal domain binding to CD3. HPN328 is currently undergoing a Phase 1/2 clinical trial, aiming to evaluate its safety, tolerability, and pharmacokinetics as a monotherapy in patients with advanced cancers expressing DLL3.
On January 9, 2024, MSD (Merck Sharp & Dohme) announced an investment of approximately $680 million to acquire Harpoon Therapeutics. With this acquisition, MSD gains access to a range of T-cell engagers in development, including HPN328, as part of its strategy to expand its oncology research and development pipeline.
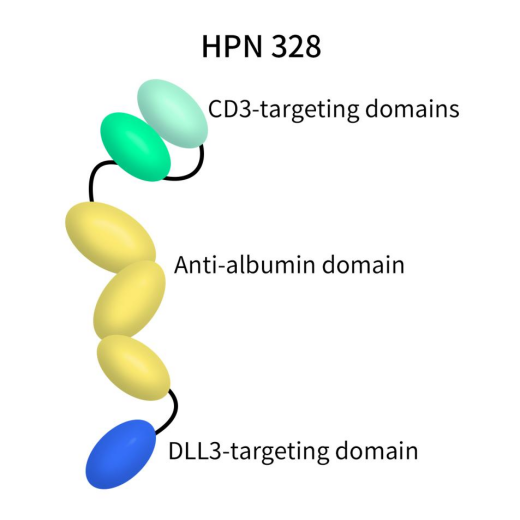
Figure 7. the structure of action of HPN328
ZG006, developed by Zelgen Pharma, is a trispecific antibody designed to target CD3 and two distinct epitopes of DLL3 (CD3×DLL3×DLL3). Preclinical studies have demonstrated significant tumor suppression effects of ZG006 in mouse tumor models, leading to complete tumor regression. The compound has shown favorable safety profiles with low toxicity in non-human primates. Currently, ZG006 is undergoing a Phase 1/2 clinical trial, focusing on Small Cell Lung Cancer (SCLC) as the targeted indication.
- 4.4 DLL3 CAR-T
AMG 119, developed by Amgen, is a Chimeric Antigen Receptor T-cell (CAR-T) therapy designed to target DLL3. The CAR structure includes domains encoding anti-DLL3 specificity, CD28 and 4-1BB co-stimulatory domains, and a CD3 intracellular domain. In December 2019, the FDA granted orphan drug designation to AMG 119. In September 2023, clinical treatment data and pharmacokinetic characteristics of AMG 119 in SCLC patients were published in the “Journal of Clinical Pharmacology.” The results demonstrated strong cell proliferation capabilities, durable cell persistence, and favorable responsiveness of AMG 119. At tested doses, the therapy exhibited clinical safety and good tolerability, without dose-limiting toxicity (DLT). Currently, AMG 119 is in Phase I clinical trials, marking a significant advancement in the exploration of CAR-T cell therapies for the treatment of DLL3-expressing malignancies.
ALLO-213, developed by Allogene, is an allogeneic Chimeric Antigen Receptor T-cell (CAR-T) therapy designed to target DLL3. As of the current information, clinical trials for ALLO-213 have not yet been initiated.
LB-2102, developed by Nanjing Legend Biotech, is a CAR-T therapy targeting two distinct epitopes of DLL3. This innovative therapy utilizes VHH antibody technology and an “armed” CAR-T technology designed to overcome inhibitory factors in the tumor microenvironment. LB-2102 carries two VHH antibody fragments recognizing DLL3 and a transmembrane protein activated by signals in the tumor microenvironment.
In June 2023, LB-2102 was granted orphan drug designation by the FDA for the treatment of SCLC. Subsequently, in November of the same year, Novartis entered into an exclusive global licensing agreement with Legend Biotech for LB-2102. This collaboration aims to further advance the development and potential commercialization of LB-2102, showcasing its promise in addressing the therapeutic needs of patients with SCLC.
- 4.5 DLL3 CAR-NK
DLL3 CAR-NK cells, developed by Tianjin Cancer Hospital, represent a groundbreaking therapeutic approach. This CAR-NK (Chimeric Antigen Receptor-Natural Killer) therapy features an scFv domain targeting DLL3, an NKG2D transmembrane domain, and a 2B4-CD3 signaling domain. The inclusion of NKG2D and 2B4-CD3 domains enhances the activation and targeting capabilities of natural killer (NK) cells against DLL3-expressing cancer cells. Currently, DLL3 CAR-NK cells are undergoing Phase I clinical trials, focusing on the recruitment of patients with relapsed/refractory ES-SCLC (Extraordinary Sensitivity-Small Cell Lung Cancer).
5. DIMA Biotech: Advancing DLL3 Biotherapy Development
DIMA Biotech is a specialized biotechnology company focusing on preclinical research products and services for actionable drug targets. DIMA Biotech now offers a comprehensive range of products and services related to the DLL3 target. The product includes active recombinant proteins, Biosimilar antibodies, and FC-validated monoclonal antibodies. Their services encompass various protein antibody customization services, antibody humanization, and affinity maturation services. To expedite the development of DLL3 biotherapy, DIMA Biotech has established a DLL3 target single B-cell seed library. This resource allows for the rapid acquisition of lead antibody molecules in as little as 28 days. Additionally, DIMA Biotech has undertaken CAR-T or ADC molecule construction and functional validation for some existing DLL3 lead antibody molecules. For specific data inquiries and further information, feel free to contact us.
- Bioactive Recombinant Proteins
Human DLL3 Protein, hFc Tag (PME100607)
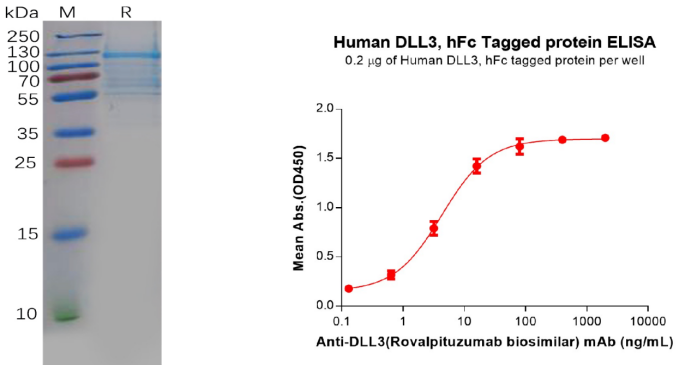
Figure 8. The validated data of PME100607. The purity of the protein is greater than 80% as determined by SDS-PAGE and Coomassie blue staining (left). ELISA plate pre-coated by 2 μg/mL (100 μL/well) Human DLL3 Protein, hFc Tag (PME100607) can bind Anti-DLL3(Rovalpituzumab biosimilar) mAb (BME100068) in a linear range of 0.64–80 ng/mL (right).
- Biosimilar Antibodies
Anti-DLL3(Rovalpituzumab biosimilar) mAb (BME100068)
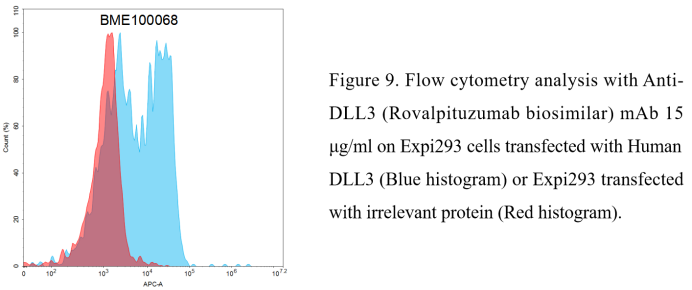
- FC-validated monoclonal antibodies
Anti-DLL3 antibody(67E6), IgG1 Chimeric mAb (DMC101089)
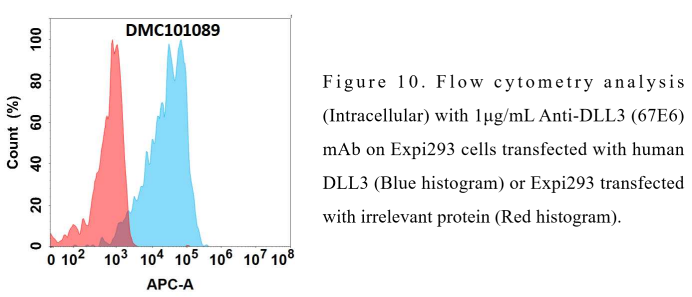
Reference:
[1]Steinbuck MP, Winandy S. A Review of Notch Processing With New Insights Into Ligand-Independent Notch Signaling in T-Cells. Front Immunol. 2018 Jun 1;9:1230.
[2]Ascano JM, Beverly LJ, Capobianco AJ. The C-terminal PDZ-ligand of JAGGED1 is essential for cellular transformation. J Biol Chem. 2003 Mar 7;278(10):8771-9.
[3]Ladi E, Nichols JT, Ge W, et al. The divergent DSL ligand Dll3 does not activate Notch signaling but cell autonomously attenuates signaling induced by other DSL ligands. J Cell Biol. 2005 Sep 12;170(6):983-92.
[4]Wang MM. Notch signaling and Notch signaling modifiers. Int J Biochem Cell Biol. 2011;43(11):1550–1562.
[5]D’Souza B, Miyamoto A, Weinmaster G. The many facets of Notch ligands. Oncogene. 2008;27(38):5148–5167.
[6]Xiu MX, Liu YM, Kuang BH. The Role of DLLs in Cancer: A Novel Therapeutic Target. Onco Targets Ther. 2020 May 7;13:3881-3901.
[7]Furuta M, Kikuchi H, Shoji T, et al. DLL 3 regulates the migration and invasion of small cell lung cancer by modulating Snail. Cancer Science. 2019 May;110(5):1599-608.
[8]Deng S M, Yan X C, Liang L, et al. The notch ligand delta-like 3promotes tumor growth and inhibits notch signaling in lung cancercells in mice[ J]. Biochemical and Biophysical Research Communi-cations,2017,483(1):488-494.
[9]Saunders LR, Bankovich AJ, Anderson WC, et al. A DLL3-targeted antibody-drug conjugate eradicates high-grade pulmonary neuroendocrine tumor-initiating cells in vivo. Sci Transl Med. 2015 Aug 26;7(302):302ra136.
