Mucin1 (MUC1), also known as MAM6 and EMA, is a surface mucin glycoprotein found to be highly expressed in epithelial-derived tumor tissues, such as lung cancer, pancreatic cancer, prostate cancer, epithelial ovarian cancer, and breast cancer. Its expression has been correlated with tumor metastasis and recurrence. Currently, MUC1 has attracted the attention of several pharmaceutical giants, including Takeda, Merck, and Qilu Pharmaceutical, all of whom have laid out pipelines for immunotherapies targeting MUC1. But what exactly is MUC1? What role does it play in the development and progression of cancer? And what types of drugs are currently being developed in clinical trials?
1. MUC1 Structure
The MUC1 gene is located on chromosome 1q22 and consists of 7 exons and 6 introns. In humans, the MUC1 gene can produce multiple subtypes through selective splicing. The MUC1 protein belongs to the mucin family and is a type I transmembrane protein, primarily comprising extracellular, transmembrane, and cytoplasmic regions. The molecular weight of MUC1 ranges from 300 to 600 kDa, making it a high molecular weight transmembrane protein composed of two subunits: MUC1-N and MUC1-C. MUC1-N and MUC1-C are linked by non-covalent bonds to form heterodimeric complexes in the cell membrane. MUC1-N (N-terminal subunit) is encoded by exons 1-3, while MUC1-C (C-terminal subunit) is encoded by exons 4-7.
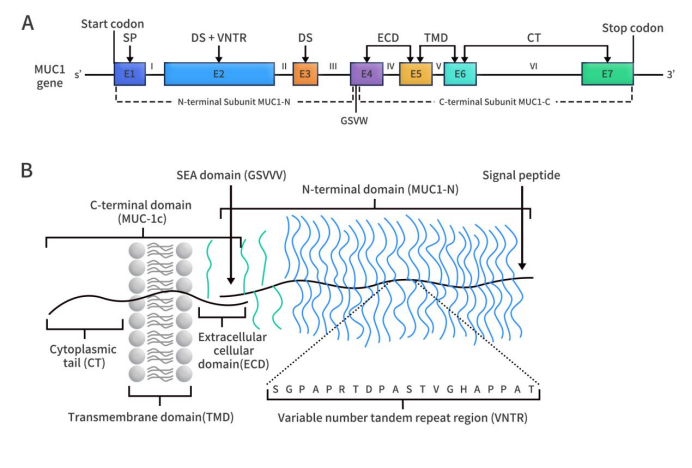
Figure 1. The structure of MUC1 gene (A) and protein (B) [1]
MUC1-N is located on the cell membrane surface and constitutes the extracellular amino acid subunit. This subunit includes a signal peptide, a variable number of tandem repeat (VNTR) region, and a SEA domain. The VNTR region consists of 12-125 repeats of a 20-amino acid sequence, with each repeat containing 5 potential O-glycosylation sites (O-glycosylation occurs at serine and threonine residues). The VNTR region is flanked by incomplete repeats (IR) sequences on both sides, which are short and similar sequences to the VNTR region, also containing serine/threonine residues, and a small amount of asparagine residues, which serve as N-linked glycosylation sites [2].
MUC1-C constitutes the C-terminal domain, including an extracellular domain (ECD), a transmembrane domain (TMD), and an unstructured cytoplasmic domain. The ECD consists of 58 amino acids, the TMD consists of 28 amino acids, and the unstructured cytoplasmic domain consists of 72 amino acids. Among these, the unstructured cytoplasmic domain contains a CQC motif, which is crucial for the formation of MUC1-C dimers and their entry into the nucleus. The ECD contains an N-glycosylation site, namely an asparagine residue. Depending on the extent of N-glycosylation, the size range of MUC1-C is 23 to 25 kDa. The molecular weight of MUC1-C lacking N-glycosylation is 17 kDa.
2. Expression and Function of MUC1
Under normal circumstances, MUC1 is widely distributed on the surface of various mucous membranes in the body, including the mammary glands, esophagus, stomach, duodenum, pancreas, uterus, prostate, and the apical surface of glandular or ductal epithelial cells in the lungs. Additionally, it is expressed to a lesser extent in hematopoietic cells [3] [4]. It acts as a lubricant and moisturizer for normal epithelial tissues, serving as a physical barrier to protect epithelial cells from the effects of external environments, pollutants, microorganisms, etc. Furthermore, it also mediates signal transduction and cell adhesion functions.
Extensive O-glycosylation and moderate N-glycosylation are the basis of MUC1’s normal biological functions. Approximately 40% of the amino acids in the VNTR are serine (Ser) and threonine (Thr) residues, which undergo extensive O-glycosylation. N-glycosylation occurs at five sites (all asparagine residues), with four located in the degenerate sequence of MUC1-N and one located in the ECD of MUC1-C. The glycosylation pattern of MUC1 varies according to the tissue-specific expression of glycosyltransferases [5]. O-glycosylation is associated with the biological characteristics of MUC1, while N-glycosylation is crucial for protein folding, sorting, secretion, and apical expression in polarized cells [6].
In normal cells, MUC1 is a highly glycosylated protein, with the peptide core being masked by glycans to protect it from enzymatic hydrolysis in the environment. Additionally, glycosylation stabilizes mucins on the cell surface by preventing mucins from undergoing meshwork protein-mediated endocytosis [7].
3. MUC1 and Tumors
In malignant tumors (such as breast cancer, gastric cancer, multiple myeloma, etc.), the biochemical characteristics and cellular distribution of MUC1 are different from those expressed in normal cells. Generally, the expression level of MUC1 in malignant tumors is more than 10 times higher than that in normal cells, and the degree of expression is directly proportional to the malignancy of the tumor. Structurally, the glycosylation of the sugar chains is incomplete and less branched in malignant tumors, leading to the formation of new sugar chain sites and exposure of peptide chain sites. This makes MUC1 susceptible to cleavage by extracellular proteases, releasing MUC1-N. The release of MUC1-N induces a conformational change in MUC1-C, thereby altering its ligand state and subsequently activating downstream cellular signaling pathways such as MAPK, PI3K/Akt, and Wnt pathways [8].
Additionally, the decrease in MUC1 glycosylation levels can also lead to decreased adhesion strength of tumor cells, providing conditions for tumor metastasis. Furthermore, the polarity distribution of MUC1 disappears, with MUC1 being expressed throughout the glandular epithelial surface and also in the cytoplasm. These MUC1 molecules are often located near growth factors and their receptors, interacting with each other. MUC1-C participates in tumor cell invasion, metastasis, and angiogenesis through intracellular signal transduction and the regulation of related biomolecules.
4. Clinical Research Progress on MUC1
Based on the role of MUC1 in tumor progression and its expression characteristics in tumor tissues, MUC1 is considered a potential target for solid tumor drug development. Currently, numerous pharmaceutical companies worldwide have developed various immunotherapies targeting MUC1, with some advancing to phase 2 clinical trials at the fastest pace. The investigational MUC1-targeted drugs include a variety of modalities such as antibody-drug conjugates (ADCs), monoclonal antibodies (mAbs), bispecific antibodies (bsAbs), vaccines, chimeric antigen receptor T cell (CAR-T) therapy, and chimeric antigen receptor natural killer cell (CAR-NK) therapy.
4.1 MUC1-ADC
DS-3939, developed in collaboration between Daiichi Sankyo and Glycotope, is an ADC utilizing Glycotope’s anti-tumor-associated MUC1 (TA-MUC1) antibody and Daiichi Sankyo’s proprietary DXd-ADC technology, with a DAR value of 8. In September 2023, Daiichi Sankyo announced that DS-3939 was undergoing phase 1/2 clinical research in patients with locally advanced, metastatic, or unresectable solid tumors (such as non-small cell lung cancer, breast cancer, urothelial carcinoma, ovarian cancer, biliary tract cancer, and pancreatic ductal adenocarcinoma), and the dosing of the first patient had been completed.
M1231, developed in collaboration between Sutro Biopharma and Merck, is a bispecific ADC targeting both MUC1 and EGFR simultaneously. M1231 utilizes Sutro’s non-natural amino acid site-specific conjugation technology, employing a cleavable Val-Cit SUTRO linker to conjugate the bispecific antibody with the cytotoxin Hemiasterlin. Hemiasterlin, a tripeptide, binds to tubulin proteins within cancer cells to exert its cytotoxicity. The bispecific antibody portion prevents mispairing of the two heavy chains through Merck’s SEED bispecific antibody technology platform. The antibody targeting MUC1 is in scFv format, while the antibody targeting EGFR is in Fab format. Clinical development of M1231 began in 2021 and is currently in phase I clinical trial (NCT04695847), targeting indications including metastatic solid tumors, esophageal cancer, and non-small cell lung cancer. According to clinical trial data, the trial is currently completed, but results have not been disclosed.
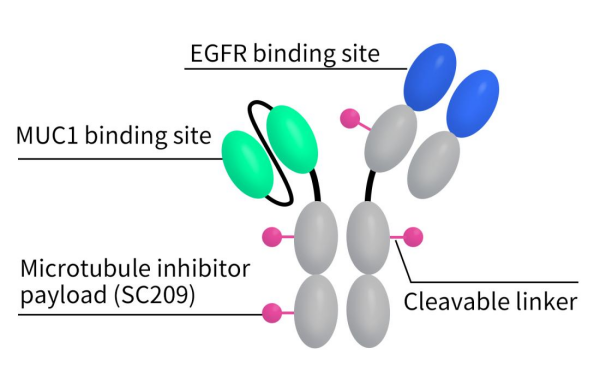
Figure 2. The structure of M1231
DXC-005, developed by Dacbiotech, is an ADC targeting MUC1. DXC-005 is composed of a Tub201 toxin small molecule (Tubulysin B analog) conjugated with an anti-MUC1 monoclonal antibody. It is the first domestically approved ADC targeting MUC1 in clinical trials. Currently, it is in the recruitment stage of phase I clinical trials for the treatment of colorectal cancer and pancreatic cancer.
BM7PE, developed by Oslo University Hospital, is an ADC drug consisting of the anti-MUC1 antibody BM7 conjugated with pseudomonas exotoxin A (PE). BM7PE is currently undergoing phase 1/2 clinical trials for metastatic colorectal cancer (NCT04550897), with the trial currently in the recruitment phase.
DM002, introduced to SinoMed from BIOCYTOGEN, is a bispecific ADC drug targeting HER3 and MUC1-C/ED. DM002 targeting MUC1-C/ED avoids the reduction in antibody-targeting tumor capabilities caused by the detachment of MUC1-N. It is currently in the preclinical stage which evaluates DM002’s safety, efficacy, and potential impact on tumor growth.
4.2 MUC1 CAR-T
Anti-MUC1 CAR-T cell (Guangzhou Anjie Biomedical) is a CAR-T therapy targeting MUC1 developed by Guangzhou Anjie Biomedical Technology Company. It is currently undergoing phase I/II clinical trials (NCT03706326) for patients with advanced non-small cell lung cancer, and the status is unknown.
huMNC2-CAR44, developed by Minerva Biotechnologies, is an autologous CAR-T cell therapy designed for solid tumors targeting the cleaved form of MUC1 present on cancer cells. Unlike the normal full-length MUC1, the cleaved form of MUC1 acts as an effective growth factor receptor. Currently, huMNC2-CAR44 is undergoing phase I clinical trials for metastatic breast cancer (NCT04020575), and the trial is still recruiting participants.
Tn-MUC1-CAR is a MUC1-CAR T cell therapy developed by Tmunity Therapeutics. Originally, Tn-MUC1-CAR was developed by a research group led by Dr. Avery D. Posey at the University of Pennsylvania. This therapy targets Tn (GalNAca1-O-Ser/Thr) and sialyl-Tn (STn) (NeuAca2-6-GalNAcal-O-Ser/Thr) glycosylated forms of MUC1. Currently, it is undergoing phase I clinical trials (NCT04025216), with the primary objective of determining the safety and phase 2 recommended dose of CART-TnMUC1 in combination with lymphodepletion. The current status is shown as terminated, with reasons undisclosed.
P-MUC1C-ALLO1 is a promising allogeneic CAR-T cell therapy developed by Poseida Therapeutics, targeting cancer cells expressing MUC1 cell surface-associated C-terminal antigen. It is currently undergoing an open-label dose-escalation phase I study (NCT05239143) targeting late-stage or metastatic solid tumors, and the trial is currently recruiting participants. In August 2023, Amgen entered into a strategic investment partnership with Poseida Therapeutics. The total investment amount is reported to be $50 million, with $25 million allocated for the exclusive negotiation rights and priority licensing for Poseida Therapeutics’ P-MUC1C-ALLO1 project.
4.3 MUC1 mAb
AR20.5, developed by OncoQuest, is a monoclonal antibody targeting MUC1, designed to activate T-cell immune responses specific to the tumor antigen MUC1, primarily for pancreatic cancer. Completed phase I clinical studies have established biological activity associated with dose and good safety.
4.4 MUC1 Vaccine
CVac, developed by Prima BioMed, is a vaccine derived from autologous monocyte-derived dendritic cells induced by mannosylated MUC1 protein. Two phase II clinical trials have been conducted with these cells: one for advanced ovarian cancer patients progressing after standard chemotherapy (NCT01521143), and another for maintenance therapy in ovarian cancer patients after clinical remission (NCT02310971). Currently, the trial NCT01521143 has been terminated, and NCT02310971 has been withdrawn.
ImMucin, developed by Hadassah Medical, is a 21-peptide vaccine containing the signal peptide domain of the MUC1 protein, capable of binding to various MHC class I and II allele genes. Currently, two clinical trials have been conducted for ImMucin: one is a phase 1/2 study evaluating the safety and efficacy of ImMucin in combination with GM-CSF in MUC1+ malignancy patients (NCT01232712), and the other is a phase II study for treating multiple myeloma patients expressing MUC1 (NCT00162500), which has been withdrawn. The NCT01232712 study demonstrated that the vaccine is safe and well-tolerated, successfully inducing vaccine-mediated cellular and humoral immune responses, with clinical disease control observed in 11 out of 15 patients.
ONT-10, developed by Cascadian Therapeutics, is a liposomal therapeutic vaccine containing two repeated components: a 20-mer synthetic glycopeptide from MUC1 and monophosphoryl lipid A (TLR4 agonist). There are currently three clinical trials ongoing for ONT-10: one is a phase Ib study investigating ONT-10 in combination with varlilumab (anti-CD27 agonistic antibody) in patients with advanced ovarian and breast cancer (NCT02270372); another is a phase I study for treating patients with solid tumors (NCT01556789); and the third is an open-label phase Ib maintenance study (NCT01978964). All three clinical trials are completed at present.
Additionally, there are other MUC1 vaccines in clinical development, including Tecemotide (L-BLP25) developed by Merck (Phase II), ETBX-061 developed by ImmunityBio (Phase II), and TG4010 developed by Transgene (Phase II).
5.DIMA Bio’s MUC1-related Products and Services
DIMA Bio is a biotechnology company dedicated to the preclinical development of drug targets and services. DIMA now offers a full range of products and services targeting MUC1, Products include active proteins, reference antibodies, and flow cytometry-validated monoclonal antibodies. Services include various species antibody customization services, antibody humanization, and affinity maturation services. Additionally, to expedite the development of MUC1 biopharmaceuticals, DIMA has prepared a library of single B cell seeds targeting the MUC1 target, with lead antibody molecules available in as little as 28 days. Furthermore, we have conducted CAR-T or ADC molecule construction and functional verification for some existing lead antibody molecules targeting MUC1. For specific data, please feel free to contact us.
- Various Species Recombinant Proteins
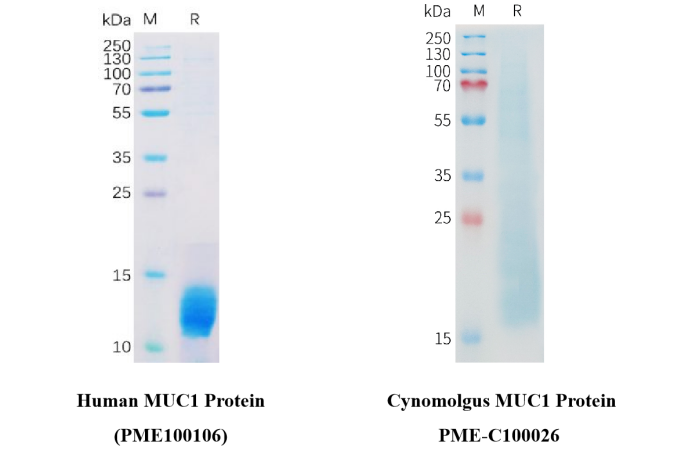
Figure 3. The purity of PME100106 is greater than 95% (left). The purity of PME-C100026 is greater than 85% (right).
- FC-validated antibody
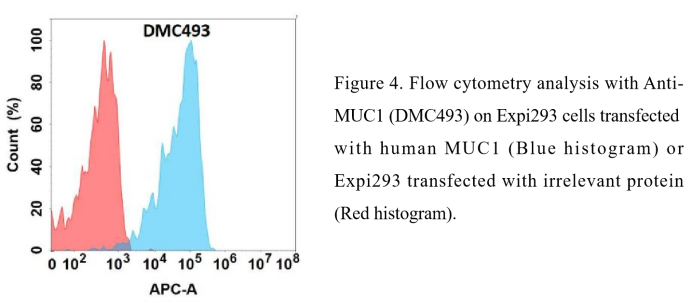
Anti-MUC1 antibody(DMC493); IgG1 Chimeric mAb ( DMC100493)
Reference:
[1]Nath S, Mukherjee P. MUC1: a multifaceted oncoprotein with a key role in cancer progression. Trends Mol Med. 2014 Jun;20(6):332-42.
[2]Tong X, Dong C, Liang S. Mucin1 as a potential molecule for cancer immunotherapy and targeted therapy. J Cancer. 2024 Jan 1;15(1):54-67.
[3]Gendler SJ. MUC1, the renaissance molecule. J Mammary Gland Biol Neoplasia. 2001;6:339–353.
[4]Chang JF, et al. The epithelial mucin, MUC1, is expressed on resting T lymphocytes and can function as a negative regulator of T cell activation. Cell Immunol. 2000;201:83–88.
[5]Hattrup CL, Gendler SJ. Structure and function of the cell surface (tethered) mucins. Annu Rev Physiol. 2008;70:431–457.
[6]Parry S, et al. N-Glycosylation of the MUC1 mucin in epithelial cells and secretions. Glycobiology. 2006;16:623–634.
[7]Altschuler Y, et al. Clathrin-mediated endocytosis of MUC1 is modulated by its glycosylation state. Mol Biol Cell. 2000;11:819–831.
Hollingsworth MA, Swanson BJ. Mucins in cancer: protection and control of the cell surface. Nat Rev Cancer. 2004;4:45–60.
