In the drug development efforts targeting complex diseases such as neuropsychiatric disorders, metabolic dysregulation, and cancer, the neuropeptide Y (NPY) receptor family is garnering increasing attention from researchers and pharmaceutical companies alike. As a typical subfamily of G protein-coupled receptors (GPCRs), NPY receptors not only regulate a variety of physiological processes but also play critical roles in the pathological mechanisms of numerous diseases, emerging as promising targets for therapeutic intervention [1,2].
This article will provide an in-depth exploration of the structure and function of the NPY receptor family, their significance in disease treatment, the current status of drug development efforts, and the major research challenges. We will also introduce how DIMA offers solutions to facilitate research in this target area.
1. NPY Receptor Family
The NPY receptors belong to the Class A GPCR family. In humans, the known subtypes mainly include Y1R, Y2R, Y4R, and Y5R. All of them are seven-transmembrane domain proteins capable of binding to NPY (Neuropeptide Y), PYY (Peptide YY), and PP (Pancreatic Polypeptide), thereby activating different downstream signaling pathways [3]:
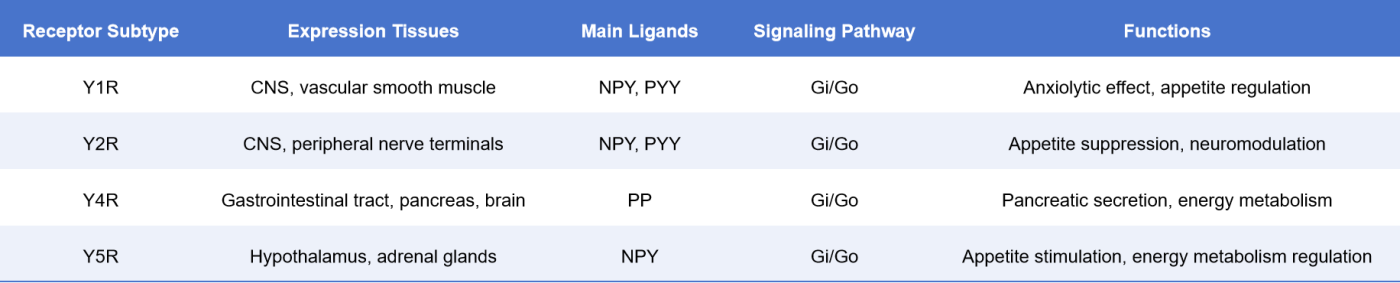
NPY receptors exhibit tissue-specific expression, with functions that are coordinated yet distinct from one another, providing an important foundation for achieving precision therapy.
2. Structure of NPY Receptors — A Typical Member of Class A GPCRs
Structurally, NPY receptors are typical members of the GPCR family with a seven-transmembrane (7-TM) helical structure. The extracellular loops are responsible for ligand recognition, while the intracellular loops interact with G proteins or β-arrestins to initiate multiple signaling pathways [2].
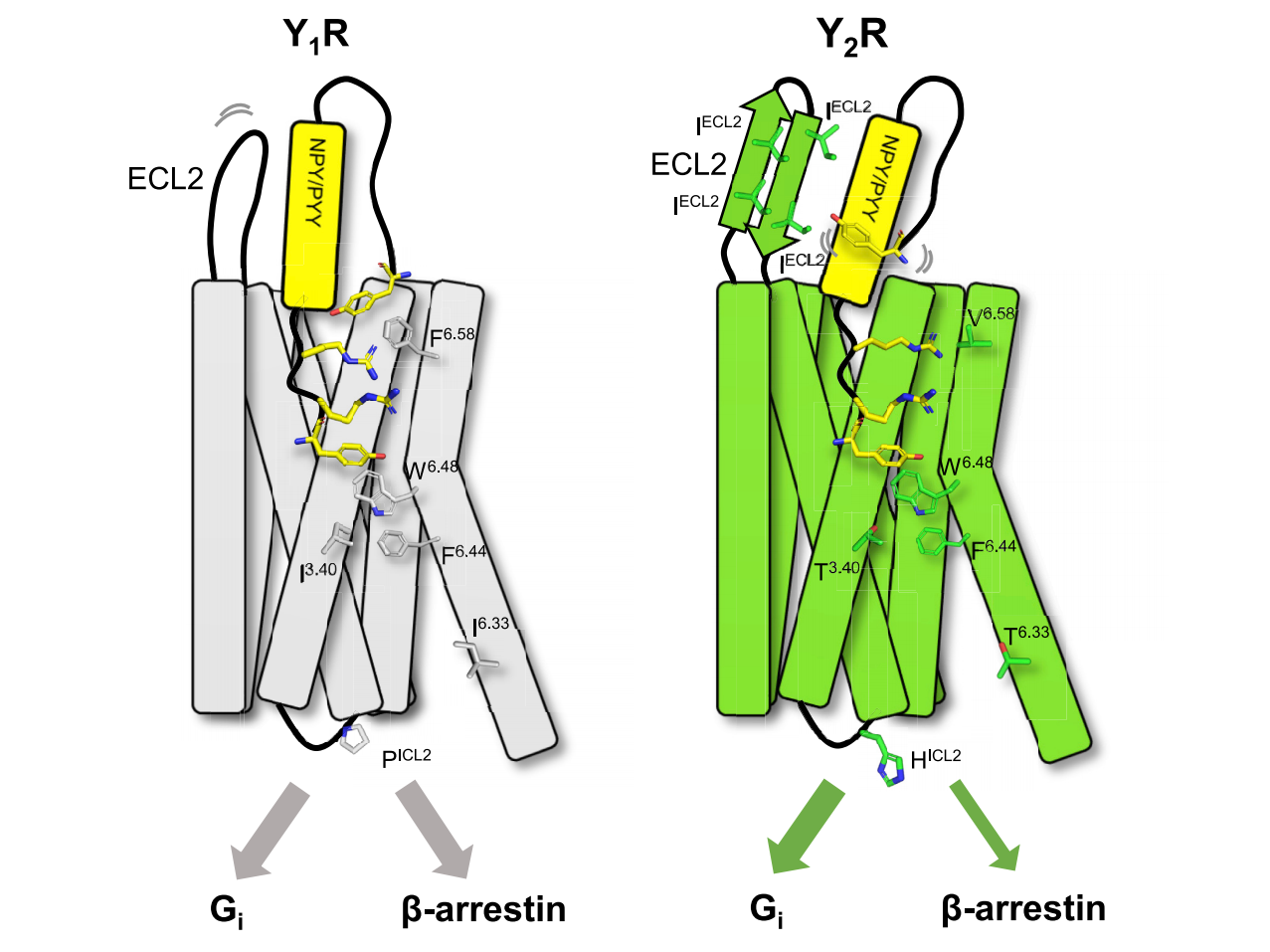
Currently, the Cryo-EM structures of Y1R and Y2R have been successfully resolved [3,4], providing templates for small-molecule drug design.
NPY receptors belong to the Class A (Rhodopsin-like) GPCRs, are encoded on human chromosome 17 (17q25.3), and consist of 381 amino acids [1]. Their basic structural features include:
- Seven-Transmembrane Structure (7-TM)
NPY receptors possess a typical seven-transmembrane helical structure, connected by three extracellular loops (ECL1–3) and three intracellular loops (ICL1–3). This framework is essential for ligand recognition and signal transduction.
- Extracellular N-terminus and Intracellular C-terminus
The N-terminus is relatively short and contains glycosylation sites, while the C-terminus harbors multiple phosphorylation sites, playing key roles in G protein coupling and receptor internalization regulation.
- Critical Amino Acid Residues
Studies have shown that acidic residues located in TM3 and TM6 are involved in ligand binding, whereas ICL2 and ICL3 are important for Gi protein coupling [2].
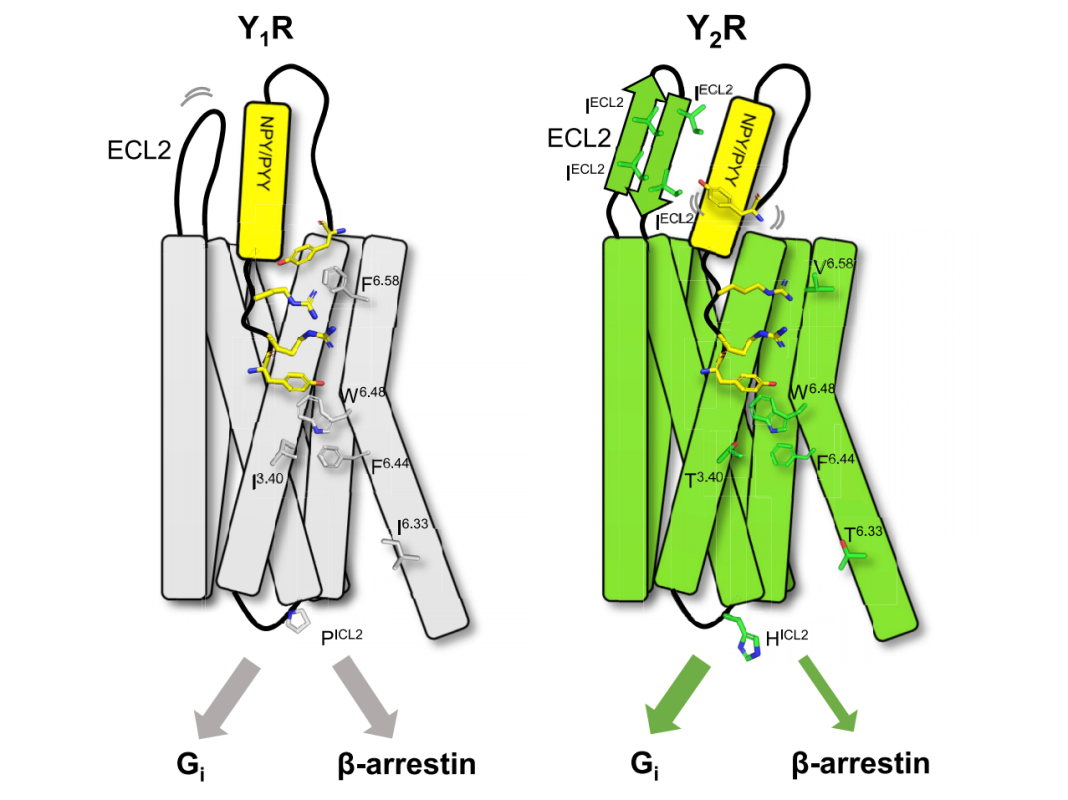
Cite from Structural basis for Y2 receptor-mediated neuropeptide Y and peptide YY signaling
3. Signaling Mechanisms of NPY Receptors
NPY receptors regulate physiological processes primarily through Gi/o protein-mediated inhibition of cAMP production, modulation of ion channels, and activation of the MAPK and PI3K/Akt pathways. Collectively, they control appetite, neuronal excitability, cell survival, and metabolic processes [4,5]. The specific mechanisms are outlined below:
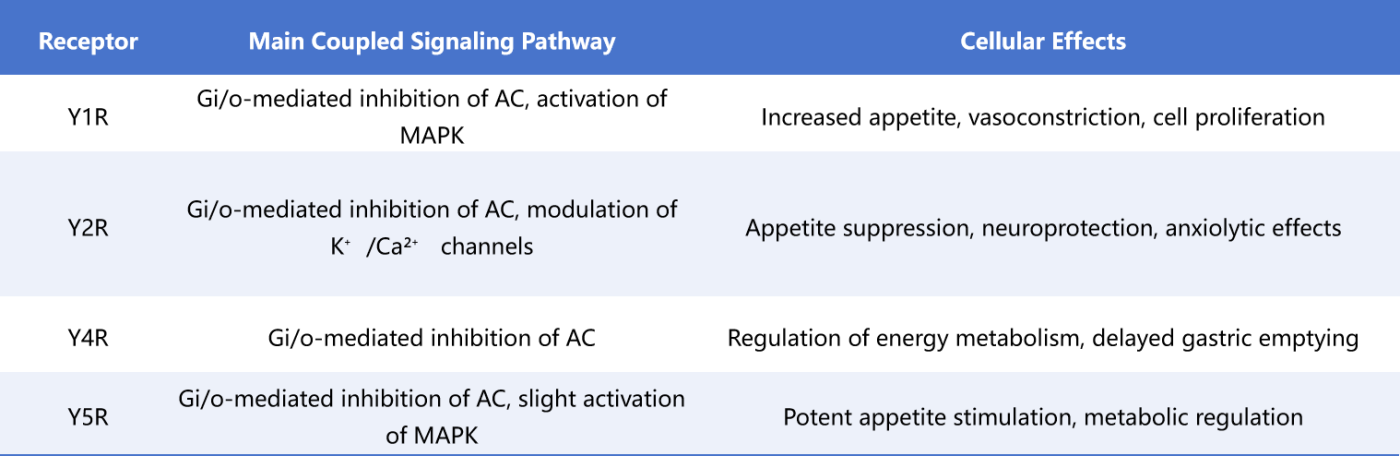
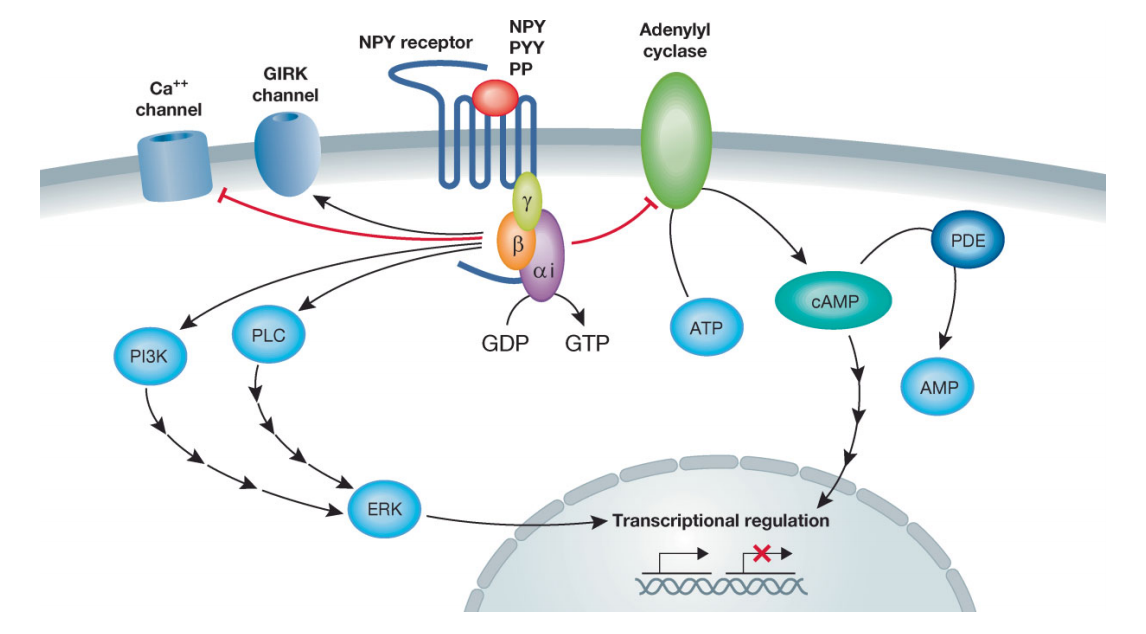
Cite from Therapeutic potential of neuropeptide Y (NPY) receptor ligands
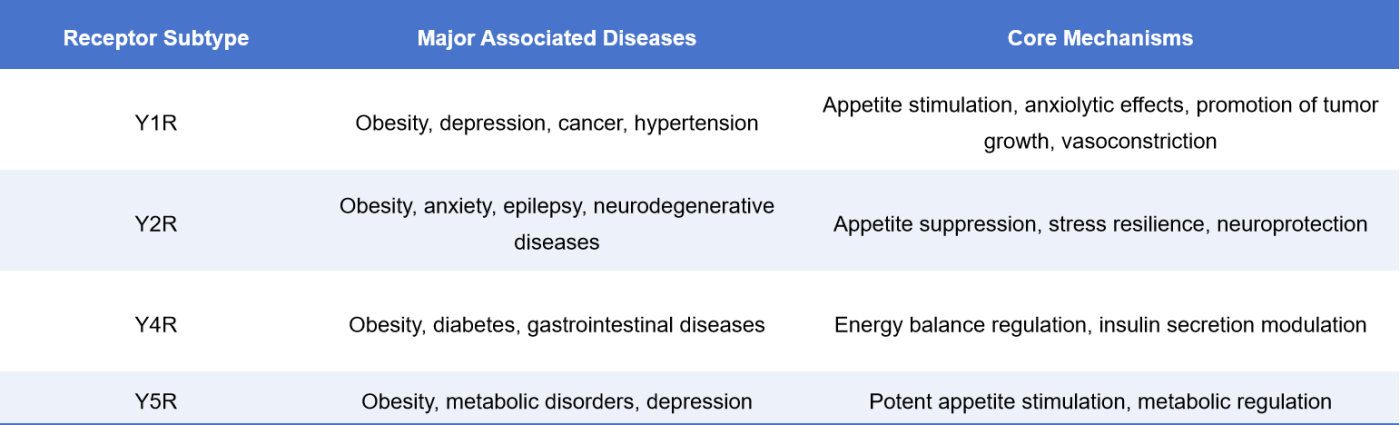
4. NPY Receptors and Disease
Within the complex neural and metabolic networks of the human body, NPY receptors act as a crucial signaling hub simultaneously regulating appetite, mood, and energy metabolism. As core components of the neuropeptide Y (NPY) system, they are highly associated with various metabolic disorders (such as obesity and diabetes) and neuropsychiatric conditions (such as anxiety and depression) [6].
4.1 Obesity and Metabolic Syndrome
The appetite-stimulating signals mediated by NPY-Y1R/Y5R receptors play a central role in the central nervous system’s regulation of energy intake. Blocking Y1R or Y5R signaling has been shown to effectively suppress feeding behavior and reduce weight gain, serving as a core mechanism in the development of several anti-obesity drugs.
- Velneperit (S-2367): A Y5R antagonist that demonstrated promising weight control potential in Phase I clinical trials (DrugBank).
- BIBP 3226: A Y1R antagonist that reduced food intake in animal studies.
4.2 Anxiety and Depression
Activation of NPY-Y1R can alleviate stress-induced anxiety behaviors, while Y2R antagonists, by lifting inhibition on Y1R, also exhibit antidepressant potential. In mouse models, activation of the NPY system has been shown to significantly improve depressive-like behaviors.
Y2R antagonists are considered promising candidates for a new generation of rapid-acting antidepressants, while Y1R agonists may be used for the treatment of PTSD.
4.3 Cancer Therapy
Y1R is highly expressed in breast and ovarian cancer cells and serves as an important tool for tumor-targeted imaging and drug delivery. Anti-cancer drug delivery systems modified with Y1R ligands can enhance tumor tissue targeting. Y1R-targeted peptide-drug conjugates (PDCs) have been developed to specifically target breast cancer cells.
Moreover, NPY receptor-targeted imaging probes have been used in PET/CT imaging for molecular imaging of tumors.
4.4 Epilepsy and Neurodegenerative Diseases
Y2R is highly expressed in the hippocampus and is associated with neuroprotection and anti-epileptic mechanisms. NPY modulates synaptic transmission through the Y2R pathway, helping to reduce excessive neuronal firing.
5. Drug Development Progress
Although NPY receptors have demonstrated clear therapeutic potential across various diseases, no NPY receptor-targeted drug has yet been successfully marketed. Most candidates remain at the preclinical or Phase I stage, primarily due to:
- Functional redundancy and cross-activity among receptor subtypes;
- Challenges in structural resolution, with very few crystal structures available;
- Lack of high-quality proteins suitable for high-throughput screening.
To date, the following NPY receptor-targeted drugs have been registered globally (data sourced from ClinicalTrials.gov and DrugBank):
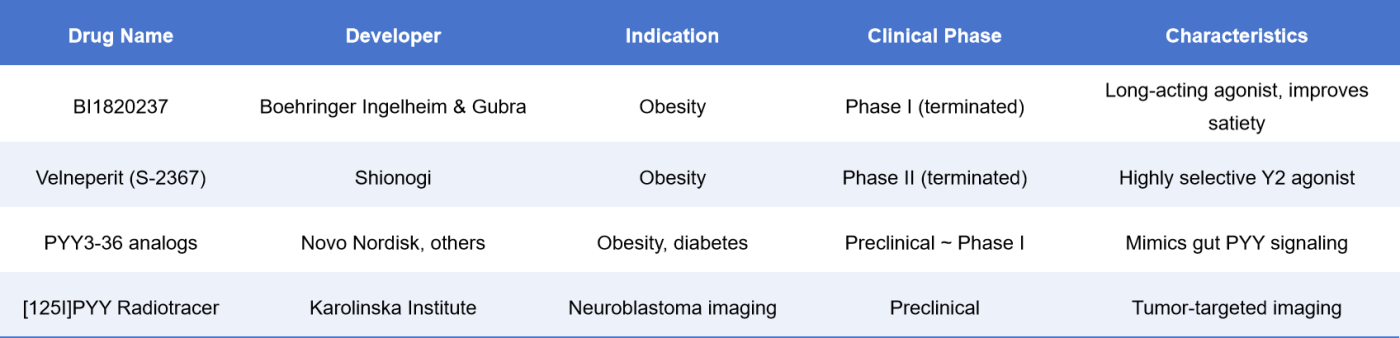
Velneperit was once the most representative Y2R antagonist in early development but was discontinued in Phase II due to insufficient efficacy [8].
PYY3-36 analogs and long-acting Y2R agonists have shown promising weight loss effects in animal studies and are currently progressing towards clinical development [9].
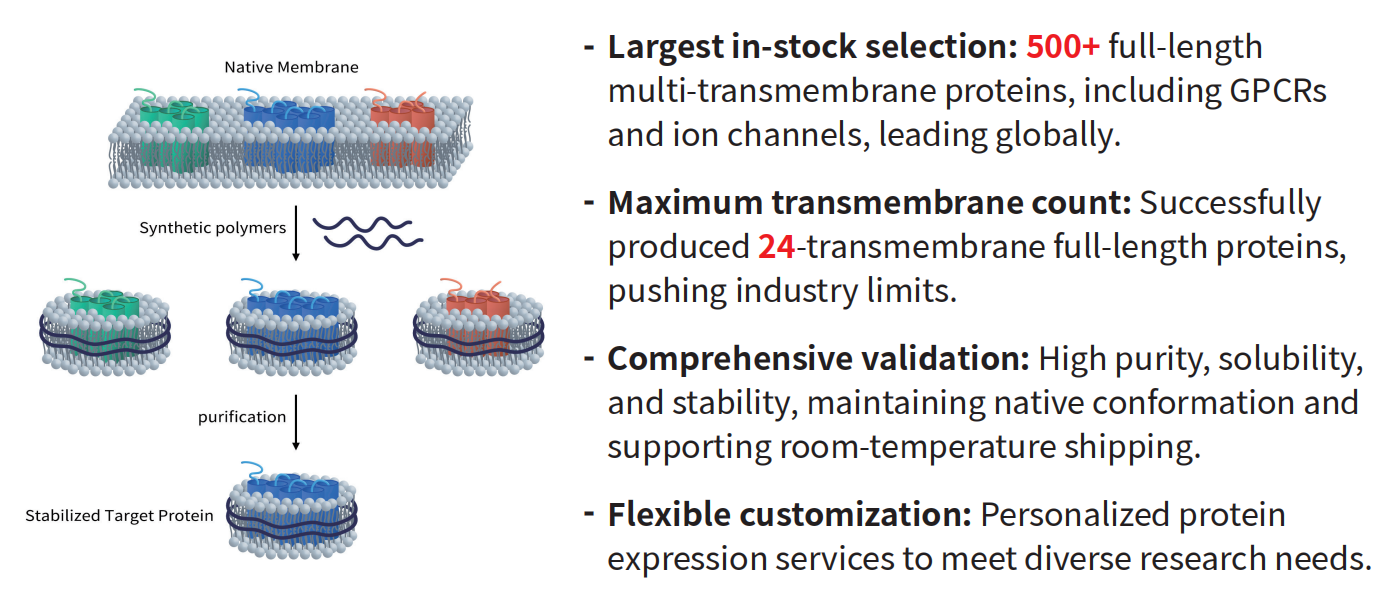
6. Nanodisc Membrane Proteins Empower Your Research
As a typical membrane protein, the seven-transmembrane structure of NPY receptors makes it extremely difficult to obtain soluble, correctly folded full-length proteins in prokaryotic expression systems. Even when expressed in mammalian cells, the proteins often exhibit low expression levels, a tendency to aggregate, and loss of functionality. This has become a major bottleneck in the following areas:
- High-affinity small molecule drug screening (HTS)
- Antibody screening and validation
- Structural determination (e.g., Cryo-EM, X-ray crystallography)
- Functional assays for signaling pathway studies
To facilitate the structural and functional research of NPY receptors and accelerate drug discovery and developability assessments, DIMA Biosciences has leveraged its innovative Synthetic Nanodisc technology platform to develop the NPY2R-Nanodisc full-length membrane protein, available as an off-the-shelf product.
Advantages of DIMA’s Synthetic Nanodisc membrane protein products include:
Data Display
Human NPY2R-Strep full length protein-synthetic nanodisc
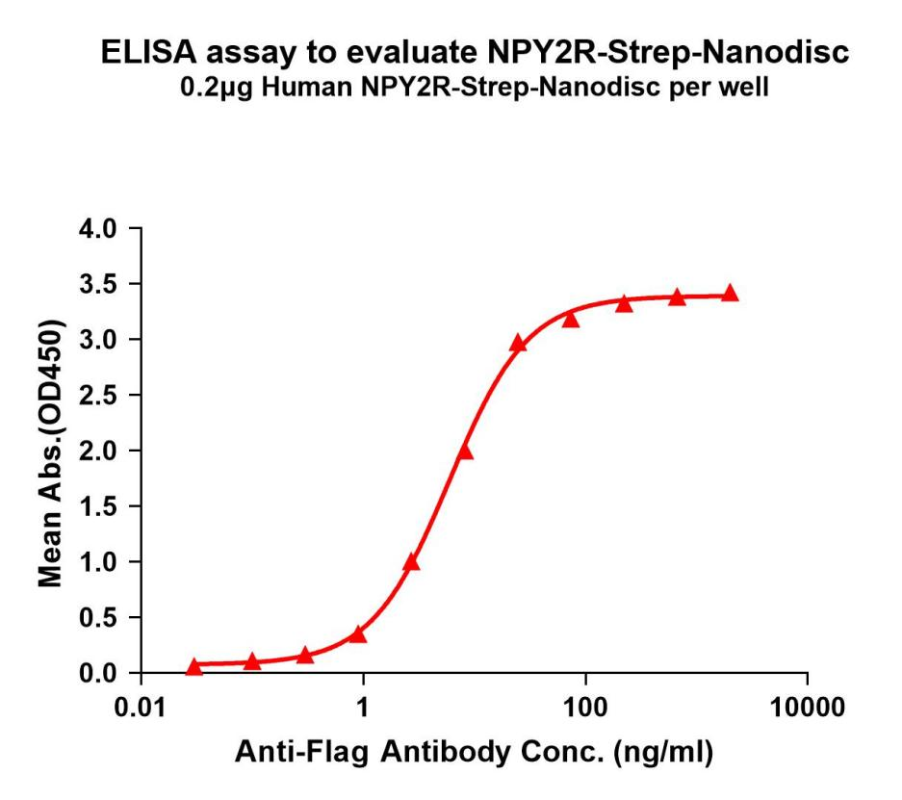
NPY2R-Strep-Nanodisc can bind with anti-Flag monoclonal antibody and the EC50 for is 5.980ng/ml.
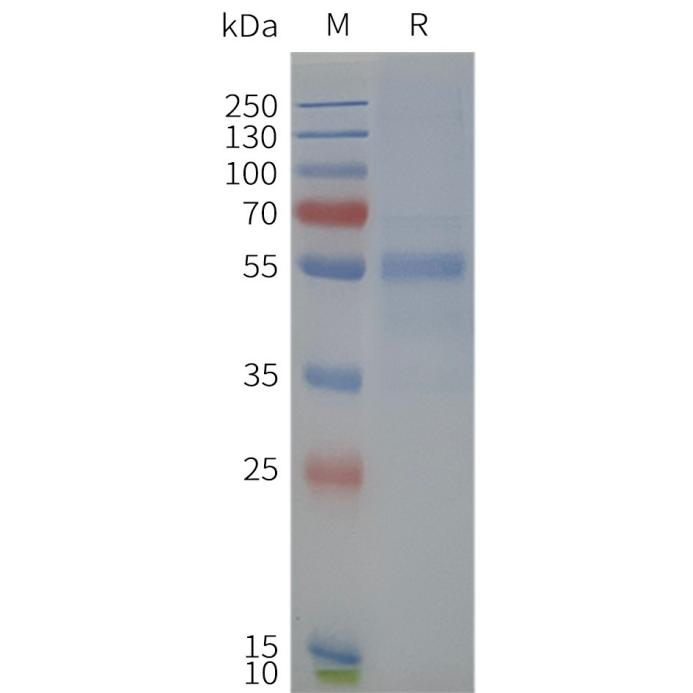
Human NPY2R-Strep-Nanodisc, C-Flag&Strep Tag on SDS-PAGE
We are committed to providing high-quality tool proteins for GPCR drug development. If you are studying NPY receptors or other GPCRs and would like to learn more, please contact us.
References
- Blomqvist AG, Herzog H. (1997). “Y-receptor subtypes—how many more?” Trends in Neurosciences, 20(7), 294–298.
- Wifling D, et al. (2015). “Pharmacological characterization of 63 NPY receptor mutants.” Molecular Pharmacology, 87(5), 778–788.
- The Human Protein Atlas.https://www.proteinatlas.org/ENSG00000185650-NPY2R
- Pedrazzini T. (2004). “NPY Y2 receptor pathways: assessment in knockout mice.” Neuropeptides, 38(6), 267–275.
- Gubra & Boehringer Ingelheim. (2023). [Company press release]
- Shionogi S-2367 Clinical Trial. https://clinicaltrials.gov/ct2/show/NCT00599406
- Holst JJ et al. (2008). “Role of PYY in regulation of food intake.” Front Neuroendocrinol.
- Shionogi & Co. – ClinicalTrials.gov ID: NCT00381888
- Batterham, R. L., et al. (2002). “Inhibition of food intake in obese subjects by peptide YY3-36.” New England Journal of Medicine, 349(10), 941–948.