Antibody-Drug Conjugates (ADCs) have emerged as a major breakthrough in oncology by combining the targeting precision of monoclonal antibodies with the potent efficacy of cytotoxic agents. Despite their success, conventional ADCs face various clinical challenges, including suboptimal efficacy, significant adverse effects, and the development of drug resistance, which together limit their broader clinical application [1]. These limitations have driven continuous innovation and optimization in ADC design.
Efforts to improve traditional ADCs have primarily focused on the three key components: the antibody, the linker, and the payload. Previously, we summarized the research progress in bispecific ADCs. Here, we further consolidate the pipeline updates of dual-payload ADCs.
1. Advantages of Dual-Payload ADCs
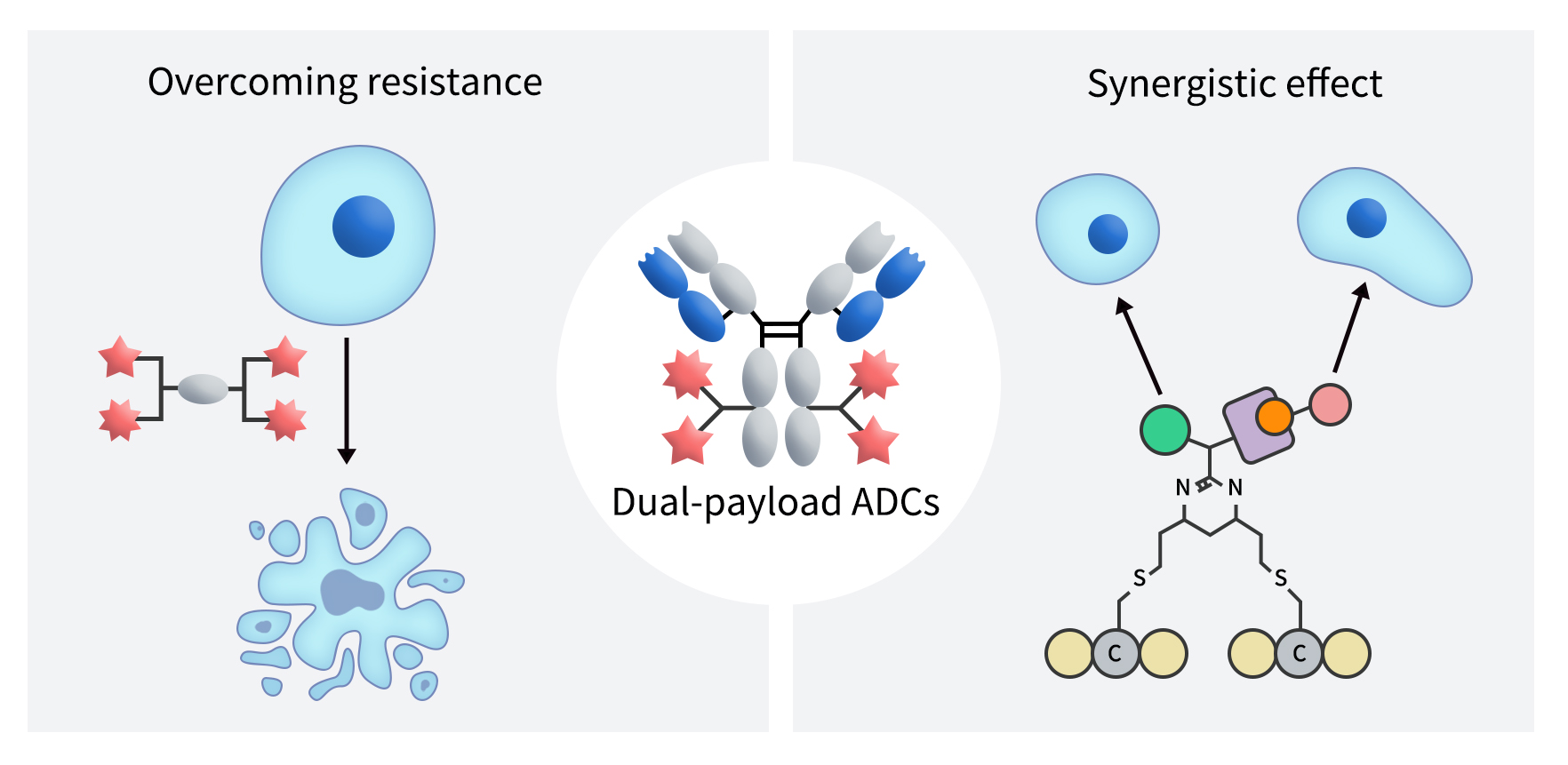
Dual-payload ADCs are engineered to conjugate two cytotoxic agents—either with similar or distinct mechanisms—onto a single antibody, aiming to overcome the limitations of traditional single-payload ADCs, enhance therapeutic efficacy, and broaden the spectrum of indications. In principle, the advantages of dual-payload ADCs can be summarized in four key aspects:
Synergistic Killing Effect: By combining two cytotoxic agents with complementary mechanisms of action, dual-payload ADCs can simultaneously target different survival pathways in tumor cells, significantly enhancing antitumor efficacy.
Overcoming Drug Resistance: By incorporating agents that tackle different resistance mechanisms, dual-payload designs can delay or even reverse the onset of drug resistance.
Expanding the Therapeutic Window: By optimizing the ratio of the two payloads, dual-payload ADCs can maintain therapeutic efficacy while reducing the toxicity associated with single-agent treatments.
Multidimensional Modulation of the Tumor Microenvironment: Some dual-payload ADCs combine cytotoxic agents with immune modulators, enabling not only direct tumor cell killing but also activation of the immune system. This promotes “immunogenic cell death,” enhancing the antitumor immune response and achieving comprehensive modulation of the tumor microenvironment.
Figure 1. The Structure of Dual-payload ADCs [1]
2. Research Progress of Dual-Payload ADCs
Overall, the development of dual-payload ADCs worldwide remains at an early exploratory stage, with only a few candidates having entered clinical trials. However, the number of players actively investing in this field continues to grow, further highlighting the increasing excitement around the dual-payload ADC track.
2.1 Progress of Dual-Payload ADCs in China
As a major force in ADC drug development, China is also actively participating in the innovation of dual-payload ADCs. In addition to well-known players such as Hengrui Pharma, DAC Biotech, Jiangsu Alphamab, and MediLink, emerging companies like Chengdu Kanghong, Affinity Biopharmaceutical, Phrontline Biopharma, and Ikeris have also entered the dual-payload ADC space.
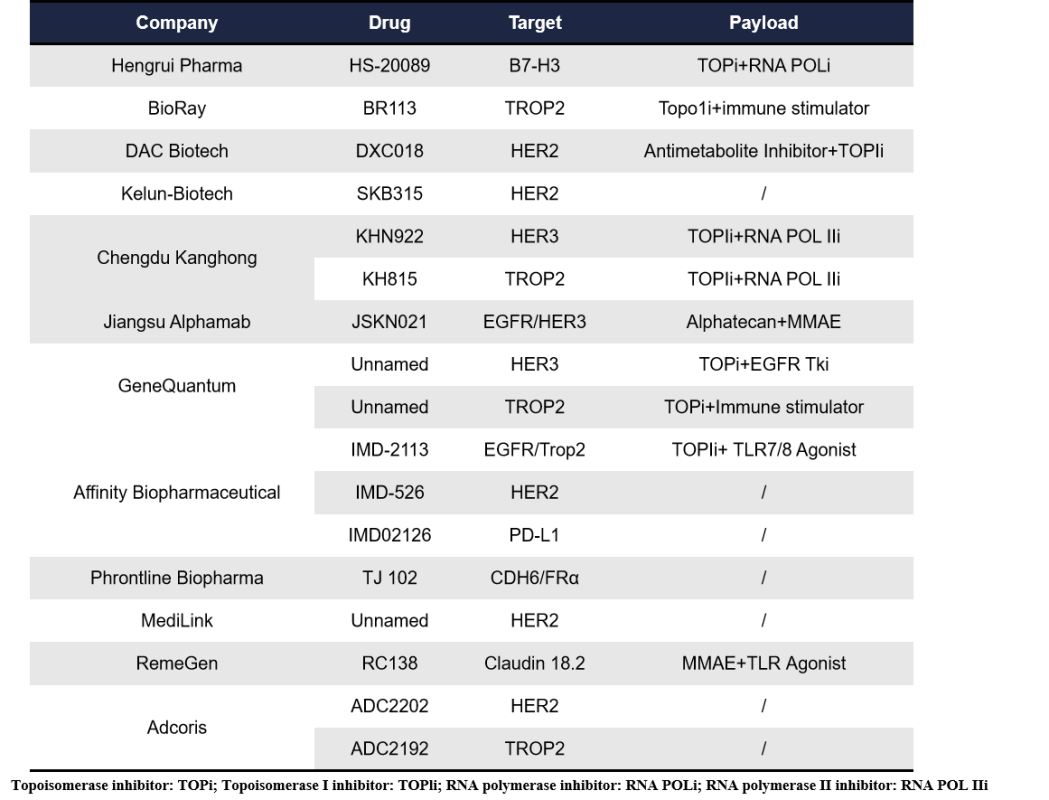
HS-20089, developed by Hengrui Pharma, is a dual-payload ADC targeting B7-H3, combining a topoisomerase inhibitor and an RNA polymerase inhibitor. In a Phase I trial for ovarian cancer, it demonstrated a disease control rate (DCR) of 70%.
DXC018, presented by DAC Biotech at AACR 2025, is a dual-epitope, dual-payload ADC targeting HER2 domains II and IV with high affinity. Its payloads include an antimetabolite inhibitor and a topoisomerase I inhibitor, effectively inducing DNA damage and cytotoxicity in preclinical studies.
SKB315, developed by Kelun-Biotech using differentiated linker technology, targets HER2. Preclinical data suggest it is effective against trastuzumab-resistant models. Kelun-Biotech also unveiled two dual-payload ADC candidates at AACR: KHN922 and KH815. KHN922 is a HER3-targeted ADC using a patritumab conjugated to a TOP1i and an RNA POL IIi. KHN922 uses cysteine and glycosylation site conjugation technology, achieving an average drug-to-antibody ratio (DAR) of ~7.5 (3.9+3.6), high SEC purity, uniform size distribution, and good thermal stability. Its dual mechanisms inhibit RNA synthesis and induce DNA double-strand breaks, demonstrating strong anti-tumor efficacy and the ability to overcome resistance in preclinical studies. KH815 targets TROP2, using the same payloads and conjugation technology as KHN922. It has also shown excellent anti-tumor activity, resistance-overcoming ability, and acceptable safety profiles in preclinical models.
JSKN021, developed by Alphamab, is a bispecific dual-payload ADC targeting both EGFR and HER3. Using cleavable linkers, it conjugates Alphatecan (a novel DNA TOPli) and MMAE to the Fc region glycan sites. JSKN021 achieved a homogeneous DAR of 4 for T01 and DAR of 2 for MMAE, and demonstrated superior tumor inhibition compared to single-payload ADCs in multiple CDX models.
GeneQuantum, leveraging its internationally leading iLDC (intelligent Large-scale Drug Conjugation) platform, has built a portfolio of 10 ADC pipelines, with five already in clinical development. In April 2023, Keymed licensed global rights (excluding Greater China) for GQ1010 to Pyramid Biosciences with an upfront payment of $20 million. In January 2025, it licensed GQ1011, a first-in-class ADC, to Biohaven for a deal valued at over $13 billion. Although GeneQuantum’s two dual-payload ADC projects have not yet disclosed their targets or names, the results are highly anticipated.
Affinity Biopharmaceutical is utilizing its proprietary Tumor Microenvironment Activated (TMEA) platform to develop multiple SMDCs, ADCs, and dual-payload ADCs. At AACR, Affinity presented preclinical data on IMD526 (HER2-ADC), IMD2126 (PD-L1-ADC), and IMD2113 (EGFR&TROP2-ADC). In various PBMC-CDX models, these dual-payload TMEA-ADCs demonstrated significant dose-dependent anti-tumor activity, complete tumor regressions, and minimal toxicity.
RC138, developed by RemeGen, is a Claudin 18.2-targeted dual-payload ADC combining MMAE and a TLR agonist. Preclinical studies for gastric cancer indicated significant immune activation effects.
Additionally, there are several other players exploring dual-payload ADCs without publicly disclosed details: Phrontline Biopharma, with a candidate named TJ102, targets CDH6 and FRα, but payload information has not been revealed. Adcoris has announced two dual-payload ADC pipelines: ADC2202 targeting HER2 and ADC2192 targeting TROP2, though the payload details are still undisclosed.
2.2 Research Progress of Dual-Payload ADCs Beyond China
In the international landscape, companies such as Adcoris, CrossBridge, Sutro, and Araris are leading the early-stage development of dual-payload ADCs, with all their pipelines currently in preclinical stages.
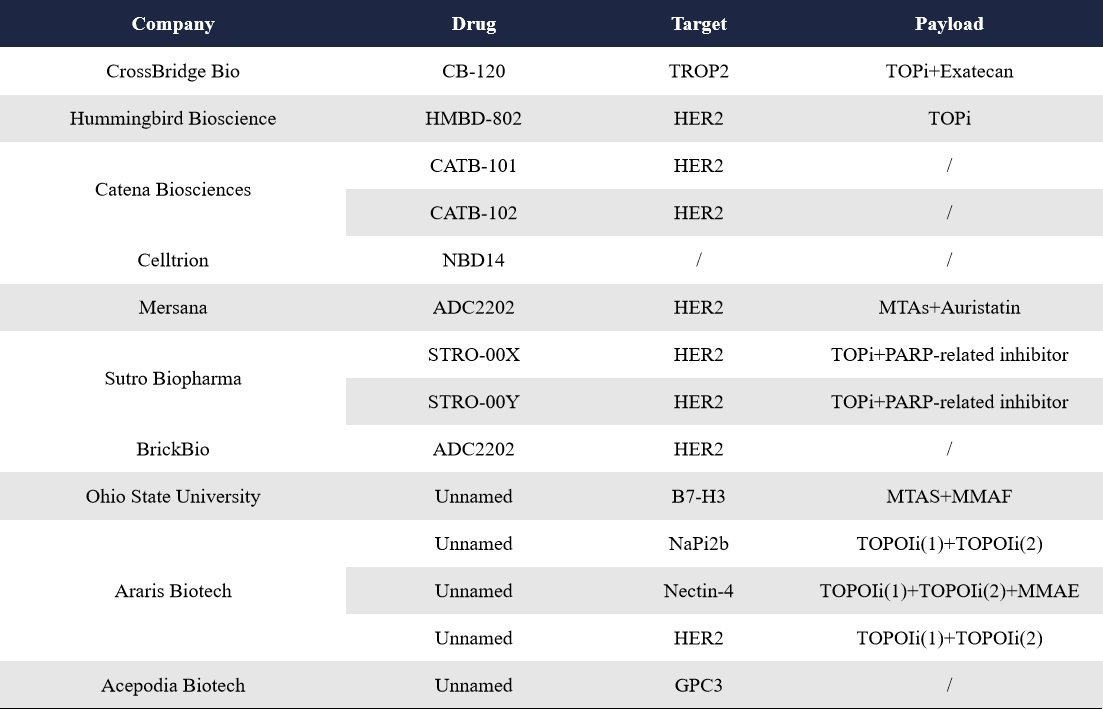
CrossBridge Bio, a U.S.-based biotech company, completed a $10 million private financing round in November last year, with a strategic focus on dual-payload ADCs. According to disclosures on its official website, CrossBridge Bio has built a pipeline of four dual-payload ADC programs. Among them, only CB-120 has been detailed so far. CB-120 is a TROP-2-targeted dual-payload ADC combining exatecan and an ATR inhibitor, and is currently the company’s most advanced ADC candidate.
HMBD-802, developed by Hummingbird Bioscience, integrates its AI-driven Rational Antibody Discovery (RAD) technology with a dual-payload linker technology via its fourth-generation ADC platform. HMBD-802 is a HER2-targeted dual-payload ADC, achieving 1:1 DAR (drug-to-antibody ratio) for the synergistic delivery of a topoisomerase I inhibitor (Topo1i) and an ATR inhibitor (ATRi), and is currently in preclinical development.
Araris Biotech stands out as one of the leading players in the dual-payload ADC space. Its innovative AraLinQ conjugation platform enables payload attachment to specific amino acid residues on antibodies without requiring antibody engineering. This efficient one-step reaction allows multiple synergistic anticancer payloads to be conjugated to a single antibody. At the 2024 AACR Annual Meeting, Araris showcased preclinical data for two dual-payload ADC programs: a HER2-targeted ADC and a NaPi2b-targeted ADC, both utilizing two different Topo1 inhibitors as payloads. The strong data helped Araris secure deals worth up to $1.92 billion. At AACR this year, Araris further presented a tri-payload ADC targeting Nectin-4, combining two different Topo1 inhibitors and MMAE. The outcome of this presentation remains highly anticipated.
Sutro Biopharma underwent major strategic restructuring in early 2025, including nearly 50% layoffs, a CEO transition, factory closures, and the discontinuation of its core drug candidate after seven years of development. Sutro is now focusing entirely on next-generation ADCs, with three preclinical projects underway, including one dual-payload ADC. Sutro disclosed two dual-payload ADC pipelines, STRO-00X and STRO-00Y, both targeting HER2 and featuring topoisomerase-based toxins combined with PARP-related inhibitors. Further details have yet to be released.
In addition to these players, other companies such as BrickBio, Catena Biosciences, Mersana Therapeutics, and Celltrion are also actively exploring dual-payload ADC technologies.
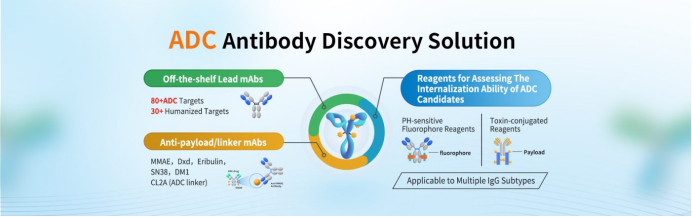
3. DIMA Biotech’s ADC Antibody Discovery Solutions
DIMA Biotechnology Co., Ltd. is a biotech company dedicated to providing products and services to biopharmaceutical companies. Unlike traditional CROs, we offer functionally validated, ready-to-use lead antibody molecules. To date, we have developed over 5,000 ready-made lead antibodies targeting more than 500 popular drug targets, all with defined antibody sequences and validated functional data. These antibodies are available for immediate acquisition and molecular testing—no waiting required.
In addition, for ADC antibody discovery, DIMA has established a comprehensive preclinical pharmacodynamic evaluation platform, specifically designed to support ADC development pipelines.
Reference:
[1] Tao J, Gu Y, Zhou W, Wang Y. Dual-payload antibody-drug conjugates: Taking a dual shot. Eur J Med Chem. 2025 Jan 5;281:116995.