Membrane proteins (MPs) play a critical role in various biological processes, such as facilitating material transport, signal transduction and enabling cell-to-cell recognition. They are frequently targeted by currently marketed therapeutics. In fact, over 60% of known drug targets specifically aim at membrane proteins such as BCMA, GPRC5D, CCR8 [1]. These proteins have been identified as key players in numerous diseases, including cancer, cardiovascular disorders, neurological conditions, and autoimmune disorders. By targeting MPs, these drugs aim to modulate their activity and restore normal cellular function, offering potential treatments for a wide range of medical conditions.
Despite the crucial roles of MPs, they have been proven to be challenging to work with, especially multi-pass transmembrane proteins, which refer to proteins with two or more hydrophobic transmembrane regions. Because of these hydrophobic transmembrane regions, multi-pass transmembrane proteins are easy to aggregate in vitro, making the isolation and purification of these proteins a technically demanding task. Additionally, their three-dimensional structure is heavily influenced by surrounding environment, making it difficult to maintain protein conformation during purification, which further complicate their study and analysis.
Instead of using extracellular domains (ECDs) as what we normally do for single-pass MPs, multi-pass transmembrane proteins require full-length proteins in order to develop functional drugs against these proteins. DIMA’s unique DiMPro Synthetic Nanodisc technology provides a promising solution for these “undruggable” targets.
- What is nanodisc?
- What are the advantages of nanodisc platform for MPs?
- What are the types of current nanodisc proteins?
- Which type of nanodisc should you use for your proteins?
- Why do we use mammalian cell expression system to produce synthetic nanodisc proteins?
- Featured synthetic nanodisc products from DIMA Biotech
1. What is nanodisc?
The concept of nanodisc was first proposed by Professor Stephen G. Sligar from University of Illinois in 2002. In the late 1990s, Professor Sligar came across the groundbreaking research conducted by Professor Ana Jonas at the same university while searching for good candidates for atomic force microscopes (AFM) structural studies. Professor Jonas’s studies focused on the predominant circulating forms of high-density lipoproteins (HDL) in the atherosclerotic process. She found that these forms typically exist as balls of variable size containing cholesterol esters, lipids, and proteins. However, she also discovered a transient form of HDL that is roughly discoidal in shape and is stabilized by the amphipathic apolipoprotein A-I (Apo-AI). Inspired by Professor Jonas’s work, Professor Sligar proposed the concept of Nanodiscs as a powerful tool for studying the structure and function of membrane proteins.
The team reconstructed larger HDL particles by removing detergents from a lipid-enriched Apo-AI fraction isolated from human blood and imaged these HDL particles on atomically flat mica [2] [3]. They found that these particles were extremely heterogeneous, with a peak in the size distribution corresponding to the length of the repeating Apo-AI protein, which indicated there was a specific size range in which these particles were commonly found [4]. To create uniform-sized nanoparticles, Professor Sligar synthesized recombinant Apo-AI coding sequence that could be expressed in in E. coli and modified the remodeling procedure. As a result of this genetic engineering and remodeling procedure, the researchers successfully produced a group of proteins called “membrane scaffold proteins” (MSPs) which can self-assemble into disc-shaped phospholipid bilayers known as Nanodiscs. These Nanodiscs were wrapped with an amphipathic helical band surrounding the phospholipid alkyl chains that can mimic the natural environment of membrane proteins and provide a stable platform for their analysis [5]. Since its introduction, Nanodiscs have revolutionized the field of membrane protein studies and become a valuable tool for drug discovery and delivery as well. Their small size, stability, and ability to mimic cell membranes make them ideal carriers for delivering therapeutic.
Nanodisc proteins are nanoscale structures composed of phospholipid bilayer and MPs. They are typically disc-shaped multi-pass transmembrane proteins with a diameter of around 10-20 nanometers. Nanodisc proteins use stabilizers, such as MSP, to provide a stable environment for incorporating the MPs. The hydrophobic face of the stabilizer interacts with the inner lipid layer, facilitating the integration of MPs into Nanodisc, which preserves the natural conformation and activity of MPs. The hydrophilic side of Nanodiscs provides high solubility and stability in aqueous solution, further enhancing their usefulness as a research tool.
Nanodiscs provide a very stable environment for MPs in vitro, making them ideal for studying ligand binding or investigating the effects of antagonists and agonists using NMR and SPR. Nanodisc proteins can also be used in various other fields, including Resonance Raman, Cryo-electron microscopy (Cryo-EM), Matrix-Assisted Laser Desorption Ionization (MALDI), protein activity studies, and time-resolved fluorescence spectroscopy. Furthermore, the assembly of antigens into Nanodiscs has shown promise in enhancing the immune response of mice, demonstrating their great potential for use in vaccine preparation and antibody production.
2. What are advantages of Nanodisc platform for MPs?
As mentioned before, membrane proteins account for more than 60% of all FDA-approved drug targets and 90% of antibody-based drug targets. To prepare functional multi-pass transmembrane protein, various cellular and cell-free expression systems have been employed. Besides Nanodisc, there are several solutions for multi-pass transmembrane protein preparation, including detergent, peptide made up of N-terminus or extracellular loops, membrane fraction, whole cell and VLP [6].
Detergent is widely used to produce multi-pass transmembrane proteins. However, one of the biggest downsides of using detergent is proteins need to be maintained in detergent-containing solutions, and detergent cannot be completely removed. This can be problematic for downstream applications, as the presence of detergent may interfere with certain experiments or affect the protein structure.
An alternative method to obtain proteins with good solubility and correct folding is to use peptides derived from the N-terminus or extracellular loops. However, the products may not resemble the structural features and conformation of the original extracellular loops of multi-pass transmembrane proteins in the native membrane.
Another option is using membrane fractions, whole cells or VLPs to maintain natural structure of multi-pass transmembrane proteins, but immunization with these products will result in high levels of nonspecific antibody background.
Comparing with these traditional solutions, Nanodisc platform offers a more advanced solution. It enables the production of full-length MPs with high purity and excellent solubility in aqueous solutions. Proteins incorporated into Nanodiscs are in native membrane environment, ensuring they remain biologically active. There is no detergent in these products, making them suitable to use in cell- based assays and other experiments.
3.What are the current Nanodisc protein types?
Nanodisc proteins have gained significant interest in recent years due to their ability to mimic the natural lipid environment and provide a stable platform for membrane proteins. There are two types of Nanodisc proteins according to different stabilizers: Membrane scaffold protein (MSP) Nanodisc and Synthetic Nanodisc proteins.
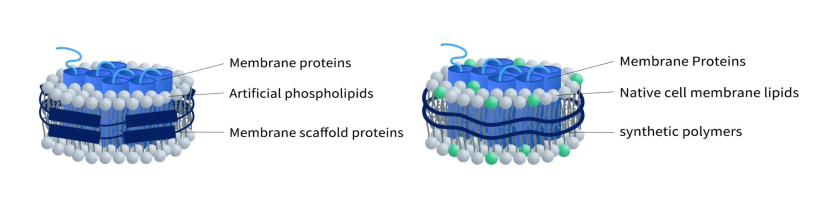
Figure 1. The structure of MSP-Nanodisc (left) and Synthetic Nanodisc (right)
3.1 MSP nanodisc
MSP nanodisc uses a specific protein called Membrane Scaffold Protein (MSP) as a stabilizer to wrap artificial phospholipid components and transmembrane proteins to form a nanodisc. This type of nanodisc is commonly used for membrane protein production before polymer Nanodisc and has been extensively studied.
This type of Nanodisc protein consists of a hydrophobic surface towards the inner lipid layer and a hydrophilic surface towards the outer layer, which allows MPs to be highly soluble in aqueous solutions even in the absence of detergents. The size of MSP Nanodisc protein is determined by the selected MSPs which usually have a diameter ranging from 7 to 13 nanometers in diameter. There are several commonly used MSPs in Nanodisc assembly, including MSP1D1, MSP1D1-DH5, and MSP1E3D1. These MSPs have been extensively studied and proven to be effective in generating stable and functional Nanodisc proteins.
The preparation of MSP Nanodisc proteins can be achieved through the following two methods
-
Assembly of Membrane Proteins Dissolved in Detergent
The first method involves the assembly of membrane proteins that have been dissolved in detergents. After the purification of membrane proteins in the presence of detergents, MSPs and phospholipids are added to the solution. The combination of membrane proteins, MSPs, and phospholipids leads to the spontaneous formation of Nanodiscs. To obtain purified Nanodisc proteins, detergents are removed through size exclusion chromatography or affinity purification. The figure below takes the A2AR protein as an example to illustrate the specific process.
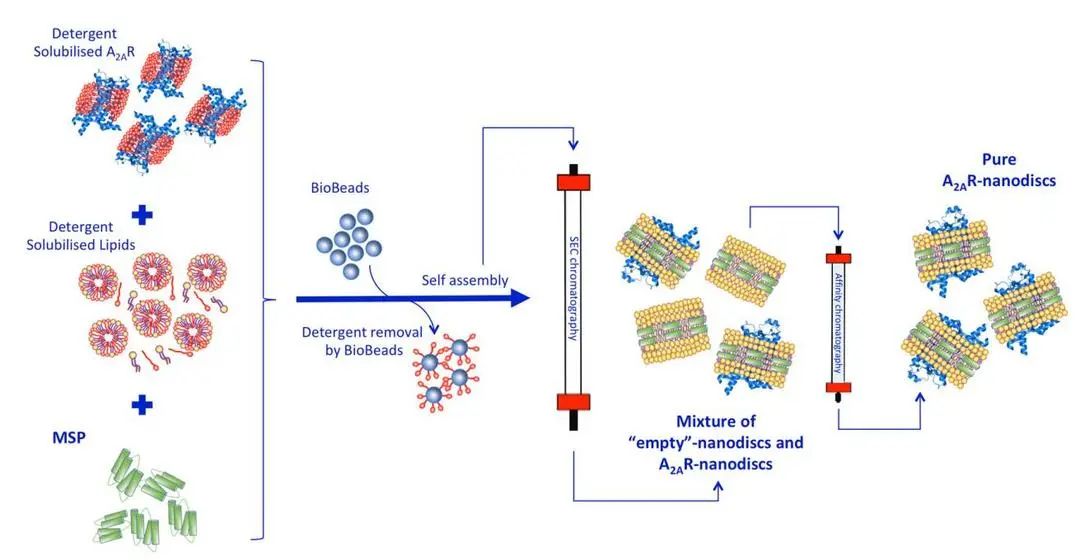
Figure 2. Assembly of MSP-Nanodisc [7]
-
Combination of Nanodisc and Cell-free expression System
Alternatively, Nanodiscs can be generated by combining them with a cell-free expression system. In this approach, pre-assembled Nanodiscs are added to a cell-free reaction system. Starting from expression plasmids, membrane proteins are produced in a cell-free system and then integrated spontaneously into Nanodiscs. This method provides a convenient way to produce Nanodiscs with specific MPs. Their hydrophobic-hydrophilic arrangement, along with the flexibility of the assembly process, makes them valuable tools in various fields of biological research.
3.2 Synthetic Nanodisc
Unlike MSP Nanodisc, synthetic Nanodisc is produced with synthetic polymers, which usually include styrene-maleic acid co-polymers (SMAs) and Diisobutylene-maleic acid (DIBMA). Each polymer has its own advantages and disadvantages. For example, DIBMA doesn’t absorb light at 280 nm, which makes it an ideal choice for experiments that involve protein analysis using spectroscopy techniques. The assembly of synthetic Nanodisc protein starts with intact cells. The long chains of synthesized polymers can be inserted into the cell membrane surrounding the desired MPs. As a result, the MPs detach from the membrane and dissolve in the polymer solution. The interaction between polymer and MPs leads to the stabilization of MPs in the newly formed Nanodisc protein (As shown in Figure 4). Overall, the use of synthetic polymers in the production of Nanodiscs offers researchers more versatility and control over the stabilization and analysis of MPs. This method allows for the creation of stable and functional Nanodisc proteins that can be utilized in a wide range of biochemical and biophysical studies.
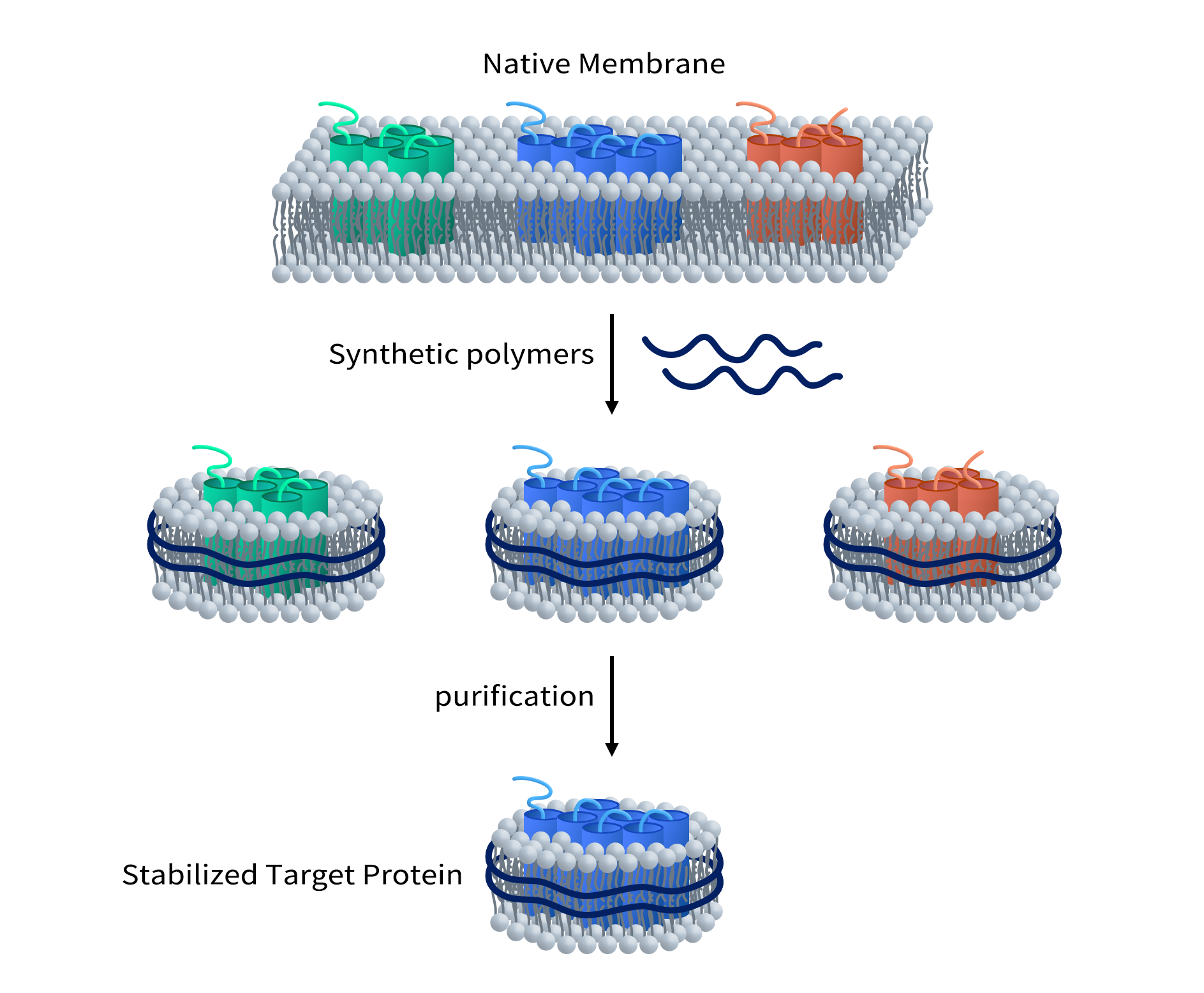
Figure 3. Assembly of Synthetic Nanodisc
4. Which type of nanodisc should you use for your proteins?
Both MSP and synthetic Nanodisc proteins are designed to achieve solubilization and stabilization of MPs. However, there are some differences between MSP Nanodisc and Synthetic Nanodisc (as shown in table).
| MSP Nanodisc Protein | Synthetic Nanodisc Protein | |
|---|---|---|
| Stabilizer | MSP | Polymer |
| Size | 7-17nm | Around 10nm |
| Lipid composition | Artificial phospholipid environments | Native cell membrane lipids |
| Protein sources | Proteins obtained from Cell-free reaction or detergent-solubilized method | Directly from the cells |
| Involvement of detergent | May use detergent during protein preparation | No detergent |
MSP Nanodisc protein is uniform in size, making it a perfect tool for applications such as Cryo-EM, whereas additional screening step may be required for the polymer Nanodisc proteins because the diameter of the polymers used for this type of Nanodisc protein is variable.
One major challenge associated with MSP Nanodisc protein is the interference caused by the presence of MSP protein. The amount of target protein can’t be easily determined by measuring the absorbance of the solution at 280nm for MSP Nanodisc protein. Moreover, MSP can lead to background noise when used together with target protein in different assays.
For MSP Nanodisc proteins, due to detergents involved in the process of dissolving the cell membrane, it is necessary to select a detergent that does not interfere with the folding of target protein. However, in Synthetic Nanodisc proteins, the polymers act as both solubilizers and stabilizers, so no detergent is required.
Because of the benefits for Synthetic Nanodisc proteins, DIMA biotech developed an innovative Synthetic Nanodisc platform for the full-length multi-pass transmembrane proteins.
5. Why do we use mammalian expression system to produce Nanodisc proteins?
All DIMA’s proteins are prepared from mammalian cell expression system. Why?
Firstly, mammalian cells are known to accurately replicate the folding and post-translational modifications of proteins, ensuring that the resulting nanodisc proteins closely resemble their natural counterparts. This is important for applications such as structural biology and drug discovery, where the native structure and function of the proteins are crucial.
Secondly, mammalian cells provide a favorable environment for complex and multi-pass transmembrane proteins. These types of proteins often require specific chaperones and co-factors for correct folding and assembly, which can be more easily achieved in mammalian cells compared to other expression systems. This ensures that the resulting nanodisc proteins exhibit the desired functionality and stability.
Additionally, the use of mammalian cells allows for the incorporation of post-translational modifications, such as glycosylation, which are important for the biological activity of many proteins. These modifications can affect the structure and function of Nanodisc proteins, making the mammalian expression system the preferred choice for producing biologically relevant proteins.
Overall, the use of the mammalian expression system in DIMA’s production of Nanodisc proteins ensures that the proteins closely mimic their natural counterparts in terms of structure, function, and post-translational modifications, making them ideal tools for a wide range of applications in biochemistry and biomedicine.
| Expression Host | Pros | Cons | Recommend Usage |
|---|---|---|---|
| E.coli | Economic Fast |
Inclusion bodies Not for large protein No PTMs |
Bacterial protein Small proteins such as cytokine and enzyme |
| Yeast | Fast Limited PTMs |
No γ-carboxylation N-glycosylation focuses on Mannose |
Small proteins such as cytokine and enzyme |
| Baculovirus | Fast Limited PTMs |
No γ-carboxylation N-glycosylation is very simple without sialic acid. |
secretory protein cytoplasmic protein toxic protein. |
| Mammalian | Diverse PTMs Good solubility |
Low yield | Complex MPs Recombinant Antibody et al. |
6. DIMA’s synthetic nanodisc
DiMProTM Synthetic Nanodisc is an innovative solution for multipass transmembrane proteins with following advantages:
- Full-length membrane proteins with high purity
- High solubility in aqueous solutions
- High stability, lyophilized powder stable at room temperature
- Proteins are in a native membrane environment and remain biologically active
- No detergent, useful for cell-based assays
- No MSP backbone proteins
The multipass transmembrane proteins produced by this technology can be used for various applications, including ELISA, SPR affinity analysis, phage display screening, Immunization, cell based assays and protein crystal structure analysis et al..
Featured Data from DIMA’s Synthetic Nanodisc Proteins
- Human MDR-1 full length protein-synthetic nanodisc (FLP100029) 12 transmembrane protein
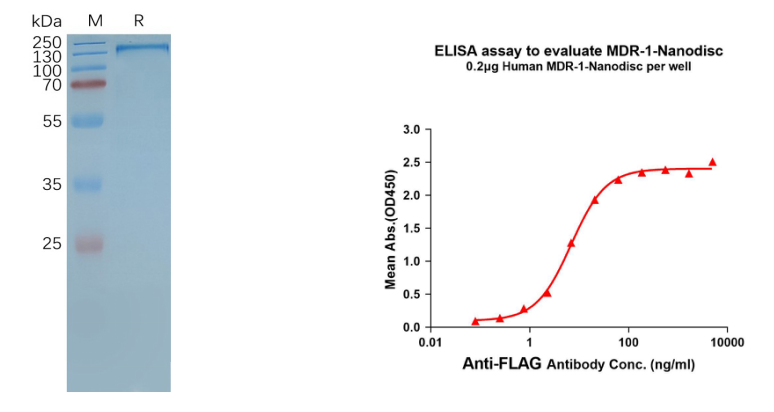
Figure 4. MDR-1-synthetic nanodisc validated data. (Left) Human MDR-1-Nanodisc, Flag Tag on SDS-PAGE; (Right) MDR-1-Nanodisc (FLP100029) can bind anti-Flag monoclonal antibody and the EC50 is 6.883ng/ml.
- Human GPRC5D full length protein-synthetic nanodisc (FLP100011) 7 transmembrane protein
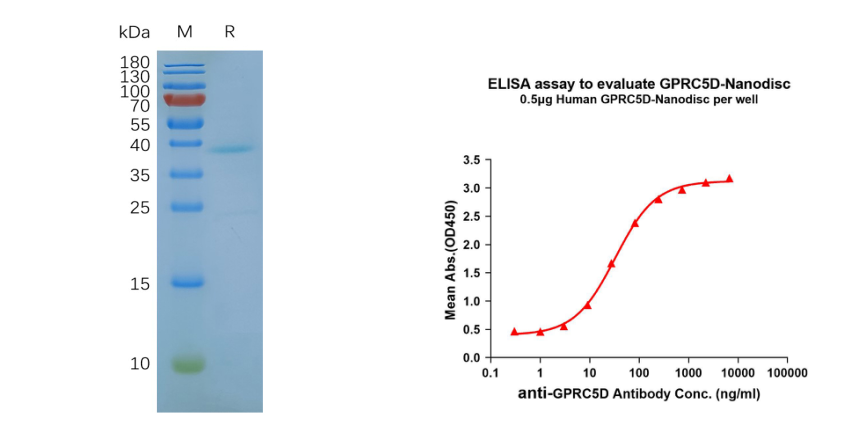
Figure 5. GPRC5D-synthetic nanodisc validated data. (Left) Human GPRC5D-Nanodisc, Flag Tag on SDS-PAGE; (Right) GPRC5D-Nanodisc (FLP100011) can bind anti-GPRC5D mAb (DME100090) and the EC50 is 32.86ng/ml.
- Human CLDN6 full length protein-synthetic nanodisc (Cat. FLP100008) 4 transmembrane protein
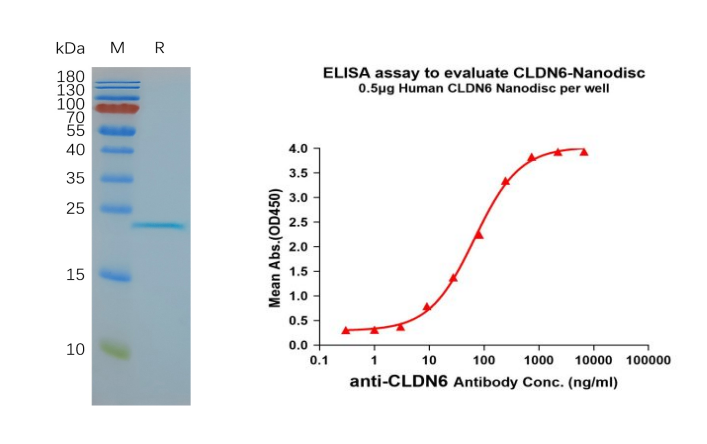
Figure 6. CLDN6-synthetic nanodisc validated data. (Left) Human CLDN6-Nanodisc, Flag Tag on SDS-PAGE; (Right) CLDN6-Nanodisc (FLP100008) can bind anti-CLDN6 mAb (BME100082) and the EC50 is 66.99ng/ml.
