The transient receptor potential ankyrin 1 (TRPA1) is a non-selective cation channel that allows the passage of Ca2+, thereby increasing intracellular Ca2+ levels. TRPA1 has garnered significant attention due to its ability to respond to various environmental stimuli and its involvement in pain perception and inflammatory processes. As of now, there are approximately 22 drugs in development targeting the TRPA1 channel globally, with 21 being small molecules and 6 having advanced to clinical trials. Recent updates on TRPA1 drug research include the notable decision by Eli Lilly in the first quarter of 2023 to discontinue a TRPA1 antagonist pain medication clinical trial. Today, we will discuss the latest clinical research advancements regarding TRPA1 drugs. Before diving into that, let’s first understand some background information on TRPA1.
- What is TRPA1?
- What is the structure of TRPA1?
- What is the mechanism of TRPA1 involved in pain?
- What is the current progress of drugs targeted TRPA1?
- The full-length TRPA1 nanodisc protein supplied by DIMA Biotechnology
1. What is TRPA1?
The transient receptor potential (TRP) protein family consists of non-selective ion channels located on the cell membrane. Based on the homology of their amino acid sequences, mammalian TRP channels are divided into seven subfamilies: TRPC, TRPV, TRPM, TRPP, TRPML, TRPA, and TRPN [1]. Transient receptor potential ankyrin 1 (TRPA1), also known as ANKTM1, is the sole member of the TRPA subfamily. It is a non-selective cation channel permeable to Ca2+ and plays a significant role in various types of pain. TRPA1 is predominantly expressed in primary sensory neurons within the trigeminal ganglion, vagal ganglion, and dorsal root ganglion, as well as in non-neuronal cells such as macrophages, dendritic cells, T lymphocytes, neutrophils, and mast cells [2]. Additionally, research has shown that TRPA1 is expressed in both peptidergic neurons (rich in neuropeptides CGRP and SP, as well as neurotrophin receptor TrkA) and non-peptidergic neurons (co-expressing purinergic receptor P2X3, Neurturin, Artemin, G protein-coupled receptors from the Mrg family, and receptors from the GDNF receptor family, such as GFRα1 and GFRα2) [3]. In recent years, TRPA1 expression has also been found in non-neuronal cells, including cochlear hair cells, endothelial cells, dental pulp fibroblasts, keratinocytes, and pancreatic islet cells [4].
2. What is the structure of TRPA1?
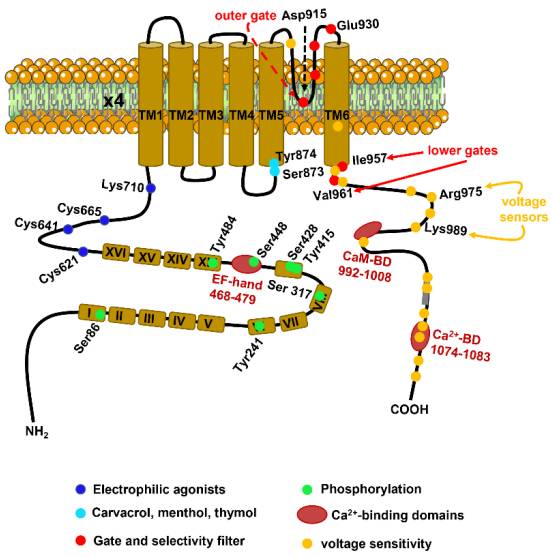
The TRPA1 gene was cloned from lung fibroblasts in 1999. Human TRPA1 consists of 1,119 amino acids and is located on chromosome 8q13, with a relative molecular mass of approximately 127 kDa. As illustrated in Figure 1, the TRPA1 transmembrane protein comprises six transmembrane domains (S1-S6) and one pore-forming region, with both the N-terminus and C-terminus located intracellularly. The S1 and S2 domains form the extracellular loop structure, while the hydrophilic regions of the S5 and S6 transmembrane domains create the pore. The long NH2-terminal of the TRPA1 protein contains the most common ankyrin repeat domain (ARD) within the TRP superfamily, consisting of 14-16 ankyrin repeat sequences. Each ankyrin repeat sequence is composed of an approximately 33-amino-acid length α-helix-β-turn-α-helix motif [5], which may be involved in protein-protein interactions, such as with phospholipase C (PLC) and calcium (Ca2+). The ARD connects to TM1 through the pre-S1 region, which includes cysteine residues (e.g., Cys621, Cys641, and Cys665) critical for the activation of TRPA1 by electrophilic agonists. The N-terminus contains an EF-hand binding domain, which can increase intracellular calcium levels. TRPA1 can be activated by numerous exogenous organic small molecules such as horseradish, cinnamaldehyde, cannabis, allicin, mustard, and protons, but it does not respond to menthol.
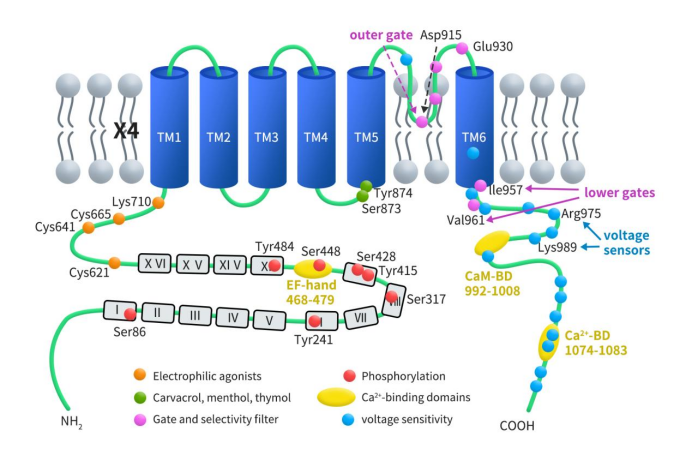
Figure1. the structure of TRPA1[6]
3. What is the mechanism of TRPA1 involved in pain?
The TRPA1 channel, discovered by 2021 Nobel Laureate in Physiology or Medicine, Professor Ardem Patapoutian, is a type of non-selective ligand-gated cation channel involved in sensing and transmitting harmful stimuli. TRPA1 can be activated by external stimuli through three distinct mechanisms: External stimuli can activate protein kinase C (PKC) via G protein-coupled receptors (GPCRs) and receptor tyrosine kinases (RTKs), thereby modulating TRPA1 channel activity; low molecular weight organic compounds and endogenous esters serve as ligands that activate the TRPA1 channel; temperature changes and mechanical stimuli directly act on the TRPA1 channel, promoting its opening and activation. Activation or sensitization of the TRPA1 channel increases Ca2+ influx, which in turn promotes neurogenic inflammation and the release of neuropeptides such as substance P (SP), neurokinin A (NKA), and calcitonin gene-related peptide (CGRP).
Multiple studies have confirmed that TRPA1 plays a significant role in mediating long-term hypersensitivity to thermal, chemical, and mechanical stimuli in both nociceptive and neuropathic pain models. McNamara CR et al. were the first to demonstrate TRPA1’s involvement in inflammatory pain perception. Their study showed that pharmacological antagonism or genetic deletion of the TRPA1 channel reduced pain responses induced by formalin in the paws of rats and mice [8]. Moreover, acute inhibition of TRPA1 in animal models induced by carrageenan and complete Freund’s adjuvant decreased cold and mechanical hypersensitivity associated with persistent inflammation [9]. Additionally, TRPA1 has been shown to contribute to both peripheral and central neuropathic pain. In streptozotocin-induced diabetic neuropathy rodent models, TRPA1 antagonists reduced mechanical allodynia and hypersensitivity [10][11].
4. What is the current progress of drugs targeted TRPA1?
Early reports in the literature identified ruthenium red, gentamicin, amiloride, and gadolinium as TRPA1 channel antagonists. However, these non-specific channel blockers have since been replaced by more selective TRPA1 antagonists. As an analgesic target within the ion channel class, TRPA1 does not carry the addiction and abuse risks associated with opioid medications. Given its position upstream in the pain signaling pathway, targeting TRPA1 drugs can block pain signals without affecting the body’s sensory modalities. Furthermore, the relatively low expression of TRPA1 in the central nervous system and heart further reduces the risk of central and cardiac side effects, offering an advantage over other analgesic targets. As mentioned earlier, there are currently six TRPA1-targeted drugs in clinical development, with one at the clinical application stage and one having received clinical approval.
| Drug Name | Company | Indication | Phase |
| GRC-17536 | Glenmark Pharmaceuticals Ltd.Ichnos Sciences SA | diabetic peripheral neuropathy | Phase II |
| LY-3526318 | Eli Lilly & Co. | Diabetic peripheral neuropathic pain, chronic pain | Phase II |
| RG-6341 | Genentech, Inc.Roche Holding AG | Refractory chronic cough, chronic cough of unknown cause, asthma | Phase II |
| HX-100 | Hydra Biosciences, Inc. | Asthma | Phase I |
| LD-2020 | Shanghai Leado Pharmatech Co Ltd | Arthritis pain, cancer pain | Phase I |
| A-967079 | Abbott Laboratories | Pain | Early Phase I |
| LD-09163 | Shanghai Leado Pharmatech Co Ltd | Inflammatory bowel disease, ulcerative colitis, Crohn’s disease | Clinical application approval |
| LD-04185 | Shanghai Leado Pharmatech Co Ltd | Fibromyalgia, neuralgia | Clinical application |
4.1 GRC 17536
GRC 17536 (also known as ISC 17536) is an oral small molecule TRPA1 channel antagonist currently being developed by Glenmark Pharmaceuticals. Preclinical studies have demonstrated the efficacy of GRC 17536 in various animal models of neuropathic and inflammatory pain, including diabetic peripheral neuropathic pain, osteoarthritis pain, postoperative pain, and chemotherapy-induced pain. These results underscore the potential utility of TRPA1 antagonists in therapeutic pain management. In September 2014, Glenmark announced positive results from a Phase IIa proof-of-concept study in painful diabetic peripheral neuropathy, showing statistically significant and clinically relevant responses in patients with moderate to severe diabetic neuropathic pain. Currently, GRC 17536 is on hold by the FDA, with official materials indicating a resumption of clinical trials expected in the second half of 2019. It is worth noting that this pipeline has been removed from the Glenmark Pharmaceuticals website.
4.2 LY-3526318
As previously mentioned, LY-3526318 was initially developed by Hydra Biosciences and began clinical trials under Eli Lilly in 2018. LY-3526318 (also known as LY356318) is a small molecule TRPA1 antagonist. On October 13, 2022, Eli Lilly completed Phase II clinical trials in the U.S. and Puerto Rico for osteoarthritis, chronic lower back pain, and neuropathic pain. On October 14, 2022, Eli Lilly planned to conduct Phase I safety and pharmacokinetics trials in healthy volunteers in Japan. However, on January 9, 2023, Eli Lilly withdrew the Phase I trial recruitment in Japan prior to enrolling healthy volunteers, citing a business decision as the official reason.
4.3 RG-6341
RG-6341, also known as GDC-6599, is a TRPA1 inhibitor developed by Roche. Preclinical studies have demonstrated that GDC-6599 exhibits strong targeting effects in guinea pig models of cinnamaldehyde-induced cough and in dermal blood flow models induced by AITC. This molecule is the first oral TRPA1 antagonist to reach Phase IIa (NCT05660850) for the treatment of chronic cough.
Despite extensive research on TRPA1 across the pharmaceutical industry over the years, only GRC 17536 and RG-6341 have progressed to the endpoints of Phase II clinical trials. Merck’s CB 189625 and Orion’s ODM108 were terminated due to poor pharmacokinetics, primarily attributed to poor solubility and low bioavailability. In contrast, domestically, Lida Biotech’s TRPA1 antagonist LD-2020 is the most advanced, currently in Phase I clinical trials in China (CTR20240694). It is noteworthy that Lida Biotech also has two other TRPA1 inhibitors, LD-09163 and LD-04185, which are at the clinical application approval and clinical application stages, respectively. This makes Lida Biotech the company with the most TRPA1 inhibitor pipelines currently in China.
Early reports in the literature identified ruthenium red, gentamicin, amiloride, and gadolinium as TRPA1 channel antagonists. However, these non-specific channel blockers have since been replaced by more selective TRPA1 antagonists. As an analgesic target within the ion channel class, TRPA1 does not carry the addiction and abuse risks associated with opioid medications. Given its position upstream in the pain signaling pathway, targeting TRPA1 drugs can block pain signals without affecting the body’s sensory modalities. Furthermore, the relatively low expression of TRPA1 in the central nervous system and heart further reduces the risk of central and cardiac side effects, offering an advantage over other analgesic targets. As mentioned earlier, there are currently six TRPA1-targeted drugs in clinical development, with one at the clinical application stage and one having received clinical approval.
5. The full-length TRPA1 nanodisc protein supplied by DIMA Biotechnology
As previously mentioned, TRPA1 is a channel protein with six transmembrane domains. Whether for antibody drugs or small molecules, obtaining an active full-length membrane protein is crucial. DIMA Biotech has developed full-length human TRPA1 protein using its proprietary Synthetic Nanodisc membrane protein expression technology platform.
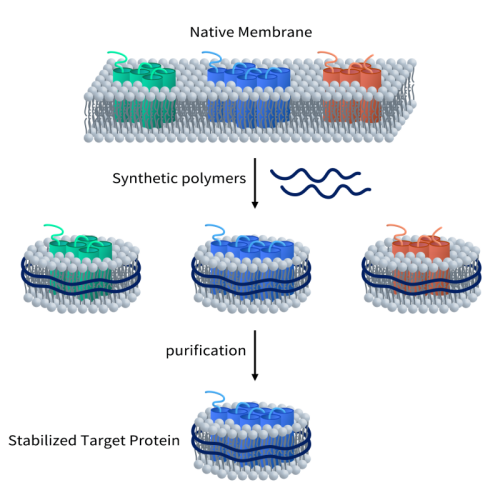
Unlike most membrane scaffold protein (MSP) Nanodiscs available on the market, DIMA’s Synthetic Nanodisc is based on an eukaryotic expression system, allowing for direct production of the full-length membrane proteins from intact cells. In this process, the synthetic polymer used serves a dual purpose. First, it dissolves the cell membrane, similar to a detergent. Then, it uses natural cell phospholipids to form a nanodisc structure around the membrane protein. The transmembrane proteins can be integrated into these Nanodiscs.
At present, DIMA Biotechnology has developed hundreds of membrane proteins using the Nanodiscs full-length membrane protein platform. Click to view all of the nanodisc proteins supplied by DIMABIO.
Human TRPA1 full-length protein-synthetic nanodisc (FLP100033)
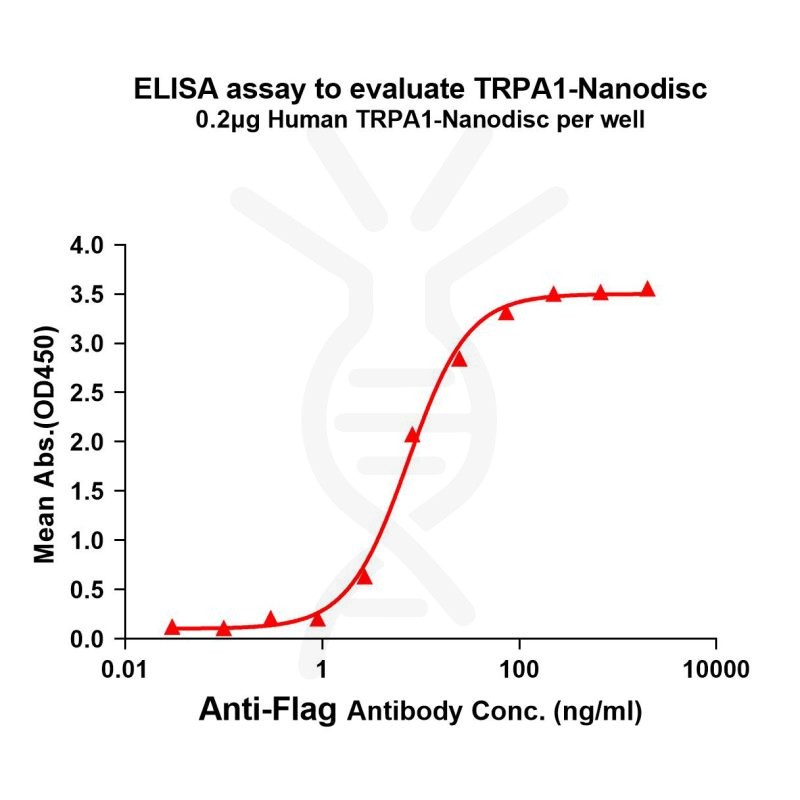
[Left] TRPA1 nanodisc protein can react with anti-Flag monoclonal antibody. the EC50 for anti-Flag monoclonal antibody binding with TRPA1-Nanodisc is 7.433ng/ml.
[Right] Human TRPA1-Nanodisc, Flag Tag on SDS-PAGE
References:
[1] Talavera K., Startek J. B., Alvarez-Collazo J., Boonen B., Alpizar Y. A., Sanchez A., et al. (2020). Mammalian transient receptor potential TRPA1 channels: From structure to disease [J]. Physiol. Rev. 100, 725–803.
[2] Iannone LF, Nassini R, Patacchini R, Geppetti P, De Logu F. Neuronal and non-neuronal TRPA1 as therapeutic targets for pain and headache relief. Temperature (Austin) [J]. 2022 May 29;10(1):50-66.
[3] KRIMON S, ARALDI D, do PRADO F C, et al. P2X3 receptors induced inflammatory nociception modulated by TRPA1, 5-HT, and 5-HT1A receptors [J]. Pharmacol Biochem Behav, 2013, 112: 49-55.[4] WILSON S R,GERHOLD K A, BIFOLCK-FISHER A, et al. TRPA1 is required for histamine-independent, Mas-related G protein-coupled receptor-mediated itch [J]. Nat Neurosci, 2011, 14(5): 595-602
[5] Paulsen C.E., Armache J.P., Gao Y., Cheng Y., Julius D. Structure of the TRPA1 ion channel suggests regulatory mechanisms [J]. Nature. 2015;520:511–517.
[6] Moccia F, Montagna D. Transient Receptor Potential Ankyrin 1 (TRPA1) Channel as a Sensor of Oxidative Stress in Cancer Cells [J]. Cells. 2023 Apr 26;12(9):1261.
[7] BONET, FISCHER L, PARADA C A, et al.The role of transient receptor potential A 1 ( TRPA1) in the development and maintenance of carrageenan-induced hyperalgesia [J]. Neuropharmacology, 2013, 65 ;206-212.
[8] McNamara CR, Mandel-Brehm J, Bautista DM, et al. TRPA1 mediates formalin-induced pain [J]. Proc Natl Acad Sci U S A. 2007;104(33):13525–13530.
[9] Bonet IJ, Fischer L, Parada CA, et al. The role of transient receptor potential A 1 (TRPA1) in the development and maintenance of carrageenan-induced hyperalgesia [J]. Neuropharmacology. 2013;65:206–212.
[10] Koivisto A, Hukkanen M, Saarnilehto M, et al. Inhibiting TRPA1 ion channel reduces loss of cutaneous nerve fiber function in diabetic animals: sustained activation of the TRPA1 channel contributes to the pathogenesis of peripheral diabetic neuropathy [J]. Pharmacol Res. 2012;65(1):149–158.
[11] Wei H, Hamalainen MM, Saarnilehto M, et al. Attenuation of mechanical hypersensitivity by an antagonist of the TRPA1 ion channel in diabetic animals [J]. Anesthesiology. 2009;111(1):147–154.