The 5T4 protein, also known as Wnt-activated inhibitory factor 1 (WAIF1) or Trophoblast Glycoprotein (TPBG), is a glycoprotein found in embryonic trophoblast cells. It has been identified as a treatment target for solid tumors due to its abundance in cancerous tissues and limited expression in normal tissues. Currently, there are approximately nine investigational drugs targeting 5T4, with nearly 20 clinical trials exploring a diverse range of therapeutic modalities, including tumor vaccines, tumor-targeted superantigen (TTS), antibody-drug conjugates (ADCs), bispecific antibodies (BiTEs), and chimeric antigen receptor natural killer (CAR-NK) cells. Among them, tumor vaccines (Trovax) and TTS (Naptumomab estafenatox) have shown significant progress, reaching Phase II clinical trials. This blog provides a comprehensive overview of 5T4, exploring its structural characteristics, signaling pathways, and clinical advancements, highlighting its potential as a versatile target for biological therapies.
1. Understanding the Structure and Expression of 5T4
The 5T4 protein is a vertebrate-specific single-pass transmembrane glycoprotein encoded by the TPBG gene, which is located on chromosome 6q14-15. Originally discovered in human placental tissues, it has a molecular weight of approximately 72 kDa and a conservative protein sequence of 420 amino acids [1]. The structure of the 5T4 protein includes an extracellular region, a transmembrane domain (356-376 aa), and an intracellular domain, illustrated in Figure 1 [2][3]. The extracellular region contains several leucine-rich repeats (LRRs) and seven N-linked glycosylation sites. The LRR regions, participating in protein-protein interactions, and the diverse structures of glycosylation sites, play a crucial role in preventing proteolysis. The intracellular region contains a PDZ interacting motif (Ser-Asp-Val), which can interact with TIP-2/GIPC, a cytoplasmic protein with a PDZ domain, associated with vesicles near cell membranes. This interaction contributes to the regulation of cell migration and adhesion signal transduction processes.
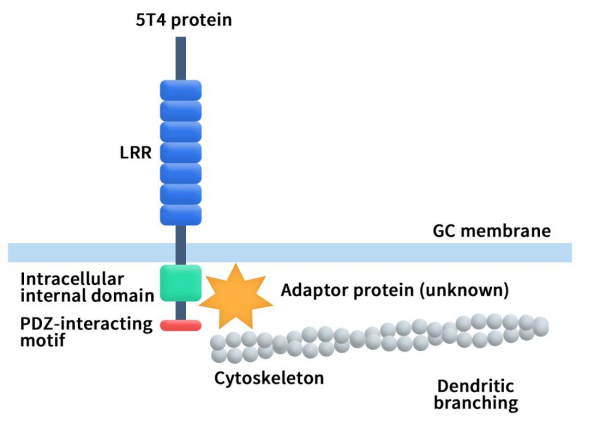
Figure 1. The structure of 5T4 [4]
While minimally expressed in normal adult tissues, 5T4 exhibits widespread expression in embryonic nourishing layer cells and is highly expressed in around 80% of renal, breast, colon, prostate, and ovarian cancers.
2. What are the Signaling Pathways of 5T4?
5T4 emerges as a promising therapeutic target for cancer treatment because of its limited expression in normal tissues and overexpression in cancer. Current research suggests that 5T4 may function through multiple signaling pathways, including the Wnt signaling pathway, epithelial-mesenchymal transition (EMT), and the CXCR4/CXCL12 pathway.
2.1 5T4 and the Wnt Signaling Pathway
A Dual ImpactThe Wnt signaling pathway plays a crucial role in embryonic development, normal cellular processes, and regeneration. It is usually divided into two types, the Wnt/β-catenin classical pathway and the non-classical pathway. The key to the Wnt/β-catenin classical pathway is the accumulation and translocation of β-catenin to the nucleus. The Wnt non-canonical pathway, also known as the planar cell polarity (PCP) pathway, is a cascade reaction caused by the binding of Wnt ligands and Frizzled receptors or their co-receptors. The Wnt signaling pathway is inactive in normal mature cells, but its activity is enhanced in tumor cells.
5T4 has been shown to interfere with Wnt/β-catenin signaling and simultaneously activate the Wnt non-classical pathway. 5T4 binds to the Wnt co-receptor LRP6 and inhibit its internalization, blocking Wnt/β-catenin signaling. Additionally, 5T4 activates the non-canonical Wnt pathway by promoting the expression of DKK1, leading to enhanced cell migration and invasion, as illustrated in Figure 2 [5].
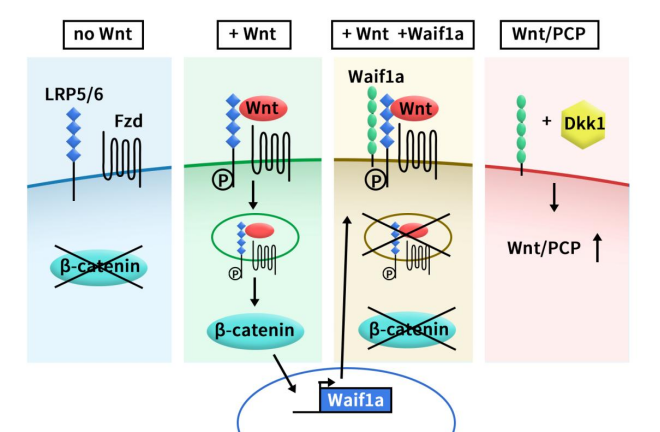
Figure 2. Model of Waif1a function as a modifier of Wnt signaling pathways [5]
2.2 Epithelial-Mesenchymal Transition: A Gateway to Invasiveness
Epithelial-mesenchymal transition (EMT) is a process wherein epithelial cells transform into mesenchymal cells, acquiring migratory and invasive capabilities. Studies indicate that 5T4 is a marker for early differentiation of mouse and human embryonic stem cells, involving classic EMT features such as the transition from E-cadherin to N-cadherin, upregulation of E-cadherin inhibitory molecules (such as Snail and Slug proteins), and enhanced activity of matrix metalloproteinases (MMP-2 and MMP-9) [6] [7] [8].
2.3 CXCR4/CXCL12 Pathway
The expression of CXCL12 and CXCR4 is associated with the occurrence of many tumors. Some studies have shown that the expression of CXCR4 helps cancer spread to tissues with high CXCL12 expression, such as lung, liver, lymph nodes, and bone marrow [9] [10]. 5T4 has been confirmed to be involved in the functional expression of CXCR4 on the surface of some embryonic and tumor cells and can promote the chemotactic metastasis of tumor cells through the CXCR4/CXCL12 pathway [11] [12].
3 What is The Clinical Progress of 5T4?
As of now, there is no globally approved targeted therapy for 5T4, with the highest development stage being Phase II. Clinical trial data indicates that 9 drugs are in various stages of development, with 5 in Phase II and 4 in Phase I (including 1 in Early Phase I).
3.1 Clinical Drugs in Phase II
Ankara-5T4 vaccine (Vaccine): Developed by SWOG Cancer Research Network, this cancer vaccine targets breast cancer and is currently in Phase II. Limited clinical effectiveness data is available.
TroVax (MAV-5T4) (Vaccine): Developed by Oxford BioMedica, TroVax is an attenuated vaccinia virus Ankara modified to encode human 5T4 antigen and kill cancer cells by stimulating the body to produce an immune response against 5T4. Clinical trials cover multiple indications, including colorectal cancer, prostate cancer, renal cancer, ovarian cancer, and mesothelioma. Clinical administration methods also involve a variety of methods, including single drugs and combinations. Combination therapies involving IL-2, Nivolumab (PD-1), or GM-CSF have been explored. A randomized clinical trial data published in 2017 demonstrated that enhanced 5T4-specific antibody immune response and prolonged OS/PFS to 20/10.3 months from 5.6/2.4 months in MVA-5T4 and combination treatment groups. Of the 55 patients with inoperable metastatic colon cancer who were stable after standard chemotherapy, 9 patients received cyclophosphamide alone, 19 patients received MVA-5T4 alone, and 18 patients received Combined treatment with MVA-5T4 and cyclophosphamide [13].
Naptumomab estafenatox (NAP) (Recombinant Protein): Developed by NeoTX Therapeutics, this fusion protein consists of an antigen-binding fragment (Fab) recognizing 5T4 and a mutated form of Staphylococcus aureus enterotoxin A (SEA/E-120). Currently, its highest research and development status is Phase II, and the indications are non-small cell lung cancer, renal cancer, and pancreatic cancer. There is a phase II/III clinical trial of the combination of NAP and IFNα in the treatment of renal cell carcinoma published in 2016. The trial data showed that the phase II/III study did not meet its primary endpoint. The median OS/PFS of patients with NAP+IFN was 17.1/5.8 months, while that of patients who received IFN alone was 17.5/5.8 months. The safety of NAP combined with IFNα is within the acceptable range [14].
ALG.APV-527 (BiTE): Developed by Aptevo Therapeutics and Alligator Bioscience AB, this bispecific antibody targets 4-1BB and 5T4. The highest development stage is Phase II, with preclinical data suggesting selective enhancement of T-cell response potential within tumors.
GEN1044 (BiTE): Developed by Genmab and AbbVie, this bispecific antibody targets CD3 on human T cells and the oncofetal antigen 5T4. Currently, in Phase II, it holds promise for immunomodulation and anti-tumor activity.
3.2 Phase I Clinical Drugs
ASN004 (ADC): Developed by Kirilys Therapeutics, this novel single-chain scFv-Fc antibody targets 5T4 using Dolaflexin-cleavable linkers coupled with auristatin derivatives (AF-HPA). Currently, it is in Phase I and targets breast cancer, non-small cell lung cancer, colorectal cancer, and ovarian cancer. Preclinical trials have shown that ASN004 can induce complete and durable tumor regression in multiple tumor xenograft models derived from human lung, breast, cervical, and gastric tumor cell lines with a wide range of 5T4 expression levels [15].
PF-06263507 (ADC): Developed by Pfizer, this antibody-drug conjugate consists of an anti-5T4 humanized antibody, a non-cleavable linker (mc), and MMAF. Currently in Phase I, it targets non-small cell lung cancer, breast cancer, and ovarian cancer. Preclinical studies confirmed its excellent anti-tumor activity. However, in the clinical phase I dose escalation trial, no ORR was observed with PF-06263507, and there was DLT mainly due to ocular toxicity (presumably caused by MMAF). Pfizer has currently terminated clinical development.
SYD1875 (ADC): Developed by Byondis B.V., this antibody-drug conjugate comprises an anti-5T4 humanized antibody, cleavable linker, and duocarmycin analog. The highest development stage is Phase I, primarily targeting solid tumors.
IBR854 (CAR-NK): Developed by Innovent Biologics, this CAR-raNK cell therapy links a specific antibody targeting 5T4 with peripheral blood-derived universal allogeneic NK cells. It has received regulatory approval for early-phase clinical trials as the first CAR-raNK cell therapy targeting solid tumors.
4. What products and services can DIMA supply?
DIMA is an innovative biotechnology company specializing in offering preclinical R&D products and services designed for druggable targets. The company has developed a range of cutting-edge platforms, including innovative functional membrane protein preparation, single B cell lead antibody discovery, and antibody engineering and functional verification platforms. DIMA can now provide a full range of 5T4 target products and services, covering active proteins from different species, -FC-verified monoclonal antibodies, and Benchmark reference antibodies. Our services include antibody humanization, affinity maturation, and PTM risk site removal. In addition, to accelerate the research and development of 5T4 target drugs, DIMA has also prepared a B cell seed library for 5T4, which can complete the screening of lead antibody molecules in as fast as 20 days.
- Recombinant proteins in various species
- ELISA&FC validated mAb
Anti-5T4 antibody(DM139); Rabbit mAb (DME100139)
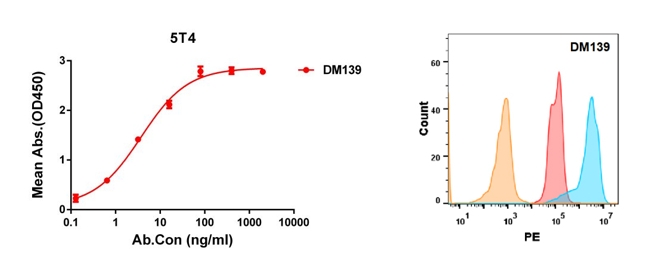
Figure 3. Validation data of Anti-5T4 antibody. Human 5T4 protein, His tagged protein can bind Rabbit anti-5T4 monoclonal antibody in a linear range of 0.1-16 ng/ml (left); 5T4 protein is highly expressed on the surface of HEK293 cell membrane (right).
More mAb:
Anti-5T4 antibody(DM138); Rabbit mAb (DME100138)
Anti-5T4 antibody(DM137); Rabbit mAb (DME100137)
- ELISA&FC validated reference antibody
Anti-5T4 reference mAb (BME100158)
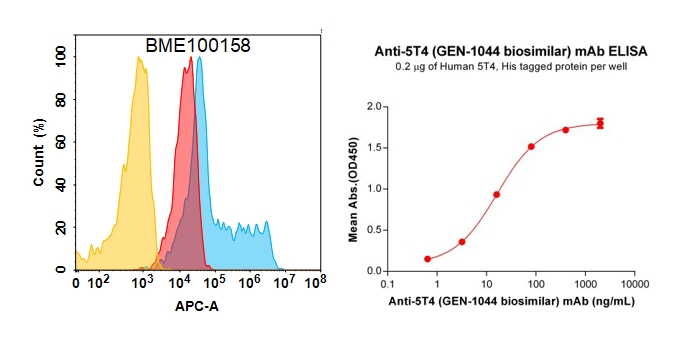
Figure 4. Validation data of Anti-5T4 reference mAb. 5T4 protein is highly expressed on the surface of HEK293 cell membrane (left); Human 5T4 Protein, His Tag (PME100123) can bind Anti-5T4 reference mAb (BME100158) in a linear range of 0.64–16 ng/mL (right).
More reference antibody:
Anti-5T4 (H8 biosimilar) mAb (BME100157)
Reference:
[1] Hole N, Stern PL. A 72 kD trophoblast glycoprotein defined by a monoclonal antibody. Br J Cancer. 1988 Mar;57(3):239-46.
[2] Myers K.A., Rahi-Saund V., Davison M.D., et al. Isolation of a cDNA encoding 5T4 oncofetal trophoblast glycoprotein. An antigen associated with metastasis contains leucine-rich repeats. J. Biol. Chem. 1994;269:9319–9324.
[3] Zhao Y, Malinauskas T, Harlos K, et al. Structural insights into the inhibition of Wnt signaling by cancer antigen 5T4/Wnt-activated inhibitory factor 1. Structure. 2014 Apr 8;22(4):612-20.
[4] Tsuboi A. LRR-Containing Oncofetal Trophoblast Glycoprotein 5T4 Shapes Neural Circuits in Olfactory and Visual Systems. Front Mol Neurosci. 2020 Oct 28;13:581018.
[5] Kagermeier-Schenk B, Wehner D, Ozhan-Kizil G, et al. Waif1/5T4 inhibits Wnt/β-catenin signaling and activates noncanonical Wnt pathways by modifying LRP6 subcellular localization. Dev Cell. 2011 Dec 13;21(6):1129-43.
[6] Ward CM, Barrow K, Woods AM, et al. The 5T4 oncofoetal antigen is an early differentiation marker of mouse ES cells and its absence is a useful means to assess pluripotency. J Cell Sci. 2003 116(Pt 22):4533–4542
[7] Ward CM, Eastham AM, Stern PL. Cell surface 5T4 antigen is transiently upregulated during early human embryonic stem cell differentiation: effect of 5T4 phenotype on neural lineage formation. Exp Cell Res. 2006, 312(10):1713–1726
[8] Eastham AM, Spencer H, Soncin F, Ritson S, Merry CL, Stern PL et al. Epithelial-mesenchymal transition events during human embryonic stem cell differentiation. Cancer Res. 2007, 67(23):11254–11262
[9] Balkwill F. The significance of cancer cell expression of the chemokine receptor CXCR4. Semin Cancer Biol. 2004, 14(3):171–179
[10] Burger JA, Kipps TJ. CXCR4: a key receptor in the crosstalk between tumour cells and their microenvironment. Blood. 2006, 107(5):1761–1767
[11] Southgate TD, McGinn OJ, Castro FV, R et al. CXCR4 mediated chemotaxis is regulated by 5T4 oncofetal glycoprotein in mouse embryonic cells. PLoS ONE. 2010, 5(4):e9982
[12] McGinn OJ, Marinov G, Sawan S, et al. CXCL12 receptor preference, signal transduction, biological response and the expression of 5T4 oncofoetal glycoprotein. J Cell Sci. 2012, 125(Pt 22):5467–5478.
[13] Scurr M, Pembroke T, Bloom A, et al. Effect of Modified Vaccinia Ankara-5T4 and Low-Dose Cyclophosphamide on Antitumor Immunity in Metastatic Colorectal Cancer: A Randomized Clinical Trial. JAMA Oncol. 2017 Oct 12;3(10):e172579.
[14] Hawkins RE, Gore M, Shparyk Y, et al. A Randomized Phase II/III Study of Naptumomab Estafenatox + IFNα versus IFNα in Renal Cell Carcinoma: Final Analysis with Baseline Biomarker Subgroup and Trend Analysis. Clin Cancer Res. 2016 Jul 1;22(13):3172-81.
[15] Smith RA, Zammit DJ, Damle NK, et al. ASN004, A 5T4-targeting scFv-Fc Antibody-Drug Conjugate with High Drug-to-Antibody Ratio, Induces Complete and Durable Tumor Regressions in Preclinical Models. Mol Cancer Ther. 2021 Aug;20(8):1327-1337.
