In recent years, the successive approvals of two new drugs have once again brought a chemokine receptor into the spotlight. In 2023, the FDA approved Motixafortide (brand name Aphexda) in combination with long-acting G-CSF for hematopoietic stem cell mobilization in patients with multiple myeloma. Then in 2024, the FDA approved Mavorixafor (Xolremdi) for the treatment of WHIM syndrome — a rare immunodeficiency disorder. Despite their distinct mechanisms of action, both drugs target the same receptor: CXCR4. This is no coincidence. It reflects the central regulatory role of CXCR4 in various physiological and pathological processes, including hematopoiesis, immune function, and tumor metastasis. In the following, we take a closer look at this critical target and the progress in drug development surrounding it.
1. Overview of CXCR4
1.1 Structure of CXCR4
Chemokine receptors form a large family of proteins that primarily mediate the chemotactic migration of cells along chemokine gradients. Among them, C-X-C chemokine receptor 4 (CXCR4) is a G protein-coupled chemokine receptor encoded on chromosome 2. CXCR4 belongs to the rhodopsin-like family and consists of 352 amino acids. Its structure includes an extracellular N-terminal domain, seven transmembrane helices, three extracellular loops, three intracellular loops, and an intracellular C-terminal domain.
On the plasma membrane, CXCR4 can exist in various oligomeric states, including monomers, dimers, higher-order oligomers, and even nanoclusters. Crystallographic studies have revealed that CXCR4 can form stable homodimers through interactions involving the TM5 and TM6 transmembrane helices, with TM6 also potentially contributing to nanocluster formation. Moreover, CXCR4 can heterodimerize with its closely related atypical chemokine receptor ACKR3 (also known as CXCR7), endowing it with unique ligand recognition and signaling regulation properties.
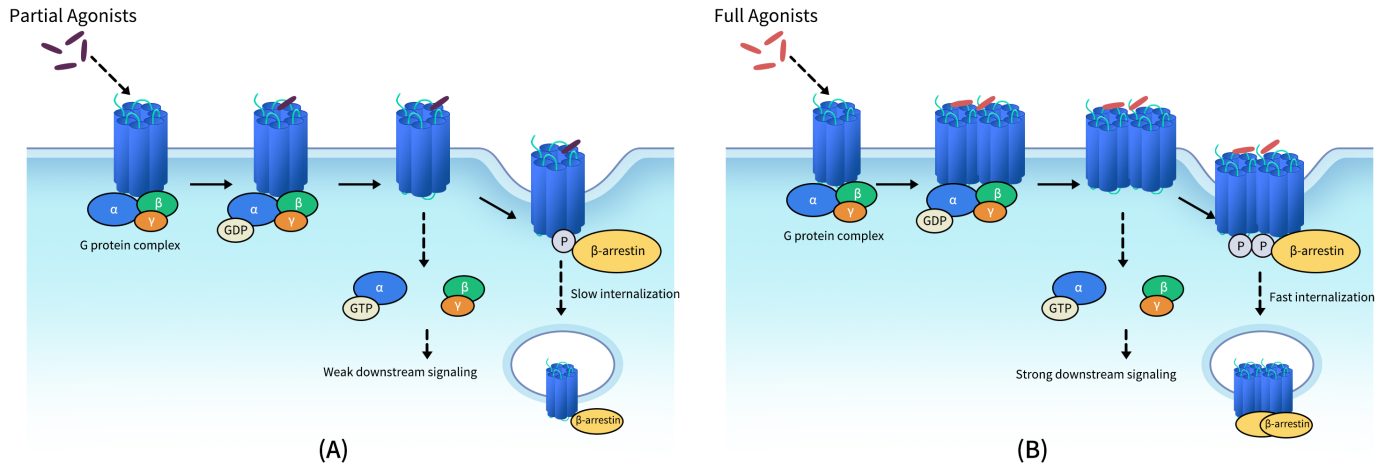
Fig 1. Schematic diagram for relationship of GPCR dimerization and their trans-membrane signal transduction functions. (A) Schematic diagram for signal tranduction of GPCRs activated with partial agonists; (B) Schematic diagram for signal tranduction of GPCRs activated with full agonists.[1]
1.2 Expression and Distribution of CXCR4
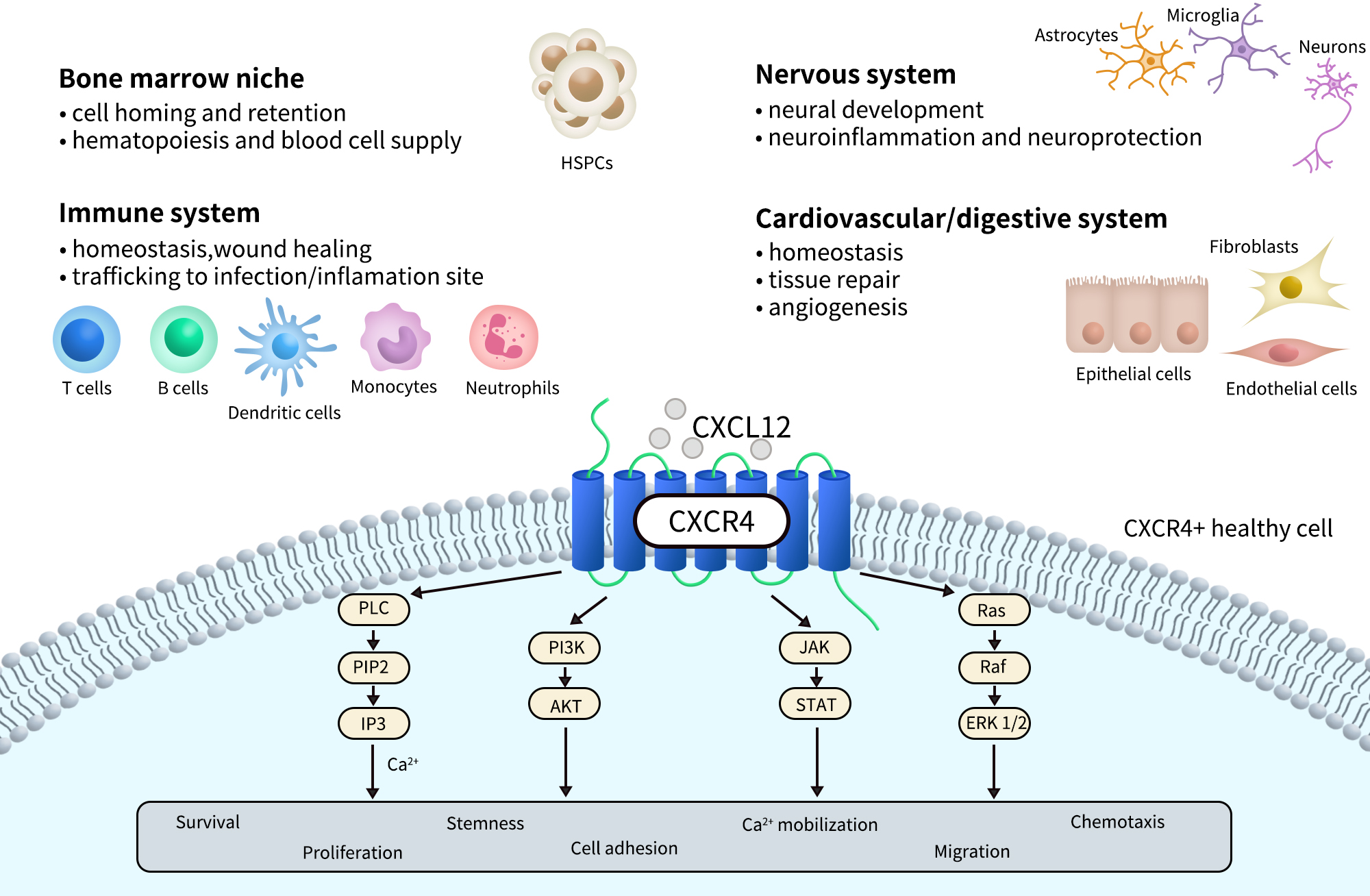
CXCR4 is widely expressed and detectable across various developmental stages and tissue types. It plays a critical role during embryonic development, particularly in the formation of the hematopoietic system, central nervous system, and cardiovascular system. In adults, CXCR4 is primarily expressed in hematopoietic stem cells, immune cells (such as T cells, B cells, and monocytes), endothelial cells, fibroblasts, and certain neurons and epithelial cells.
In addition, CXCR4 is highly expressed in a variety of tumor tissues and is closely associated with tumor metastasis, invasion, and drug resistance. As a result, it has become a key target in cancer research and targeted therapy. The natural ligand for CXCR4, CXCL12 (also known as SDF-1), is secreted by multiple tissue types and binds to CXCR4 with high affinity. This ligand-receptor interaction creates chemotactic gradients that guide directed cell migration.
1.3 CXCR4 Signaling
Upon binding to its primary ligand, CXCL12 (SDF-1), CXCR4 activates Gi-type G proteins, which in turn inhibit adenylyl cyclase (AC) activity, leading to reduced intracellular cAMP levels. In addition, CXCR4 signaling can activate a variety of downstream pathways, including PI3K/AKT, MAPK/ERK, and JAK/STAT, thereby regulating key biological processes such as cell proliferation, survival, adhesion, and migration.
Receptor activation also triggers β-arrestin–mediated internalization and biased signaling, and CXCR4 may exhibit differential signaling outputs depending on its oligomeric state (e.g., homodimers vs. heterodimers). Within the tumor microenvironment, the CXCL12/CXCR4 axis promotes immune evasion, chemotaxis of tumor cells to metastatic sites, and angiogenesis, making it a central focus in the development of immunotherapies, anti-metastatic strategies, and stem cell homing applications.
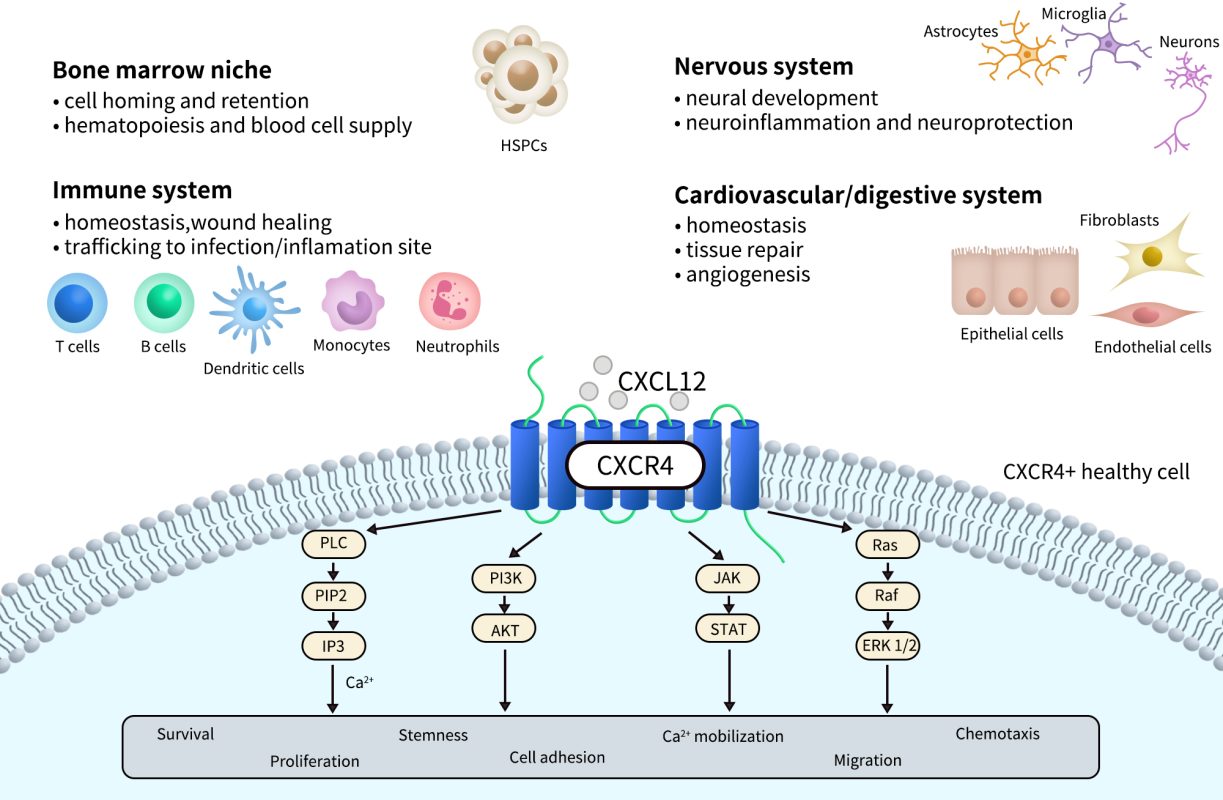
Fig 2. CXCR4 physiological and pathological processes and signaling.[2]
2. The Therapeutic Significance of CXCR4
As a classical G protein–coupled receptor (GPCR), CXCR4 plays a central role in a wide range of diseases and holds tremendous value for drug development.
2.1 A Key Player in Cancer Therapy
The CXCR4–CXCL12 axis is one of the key molecular pathways involved in tumor microenvironment remodeling and cancer metastasis.
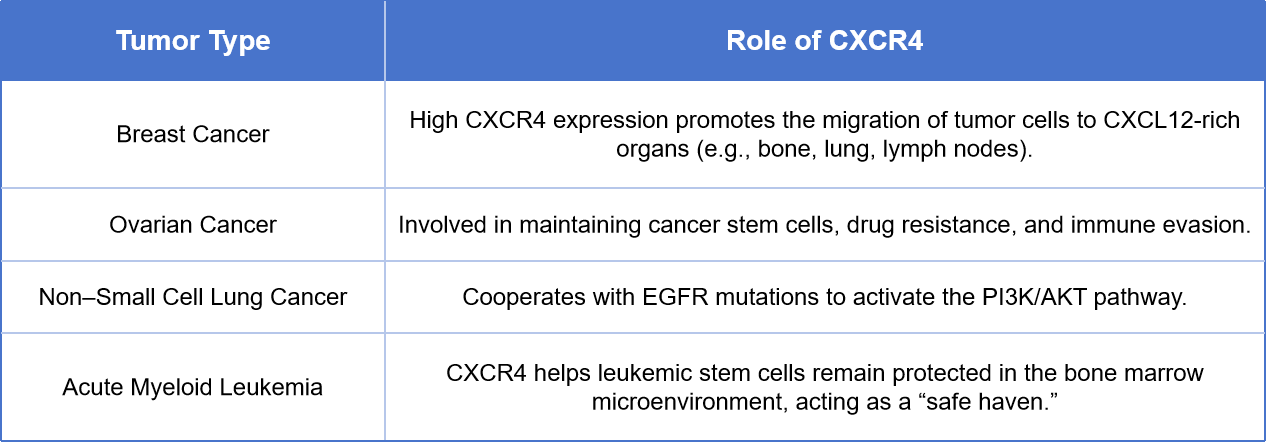
For the treatment of these cancers, current strategies involve the use of CXCR4 antagonists—such as Plerixafor and Motixafortide—to disrupt the adhesion between tumor cells and the bone marrow stroma. This approach helps restore chemosensitivity, mobilize cancer stem cells, and enhance the efficacy of immune checkpoint inhibitors.
2.2 A Regulatory Target in Immune System Disorders
CXCR4 plays a critical role in the migration, development, and localization of various immune cells, including T cells, B cells, dendritic cells, eosinophils, and neutrophils.
2.2.1 WHIM Syndrome (a Rare Disease)
WHIM syndrome is caused by nonsense mutations in the CXCR4 gene (such as R334X), which impair receptor internalization and lead to sustained CXCR4 activation. Clinically, WHIM is characterized by Warts, Hypogammaglobulinemia, Infections, and Myelokathexis—a condition where mature neutrophils are retained in the bone marrow.
In 2024, the FDA approved Mavorixafor for the treatment of WHIM syndrome, marking a successful case of targeting CXCR4 in a genetic immunodeficiency disorder.
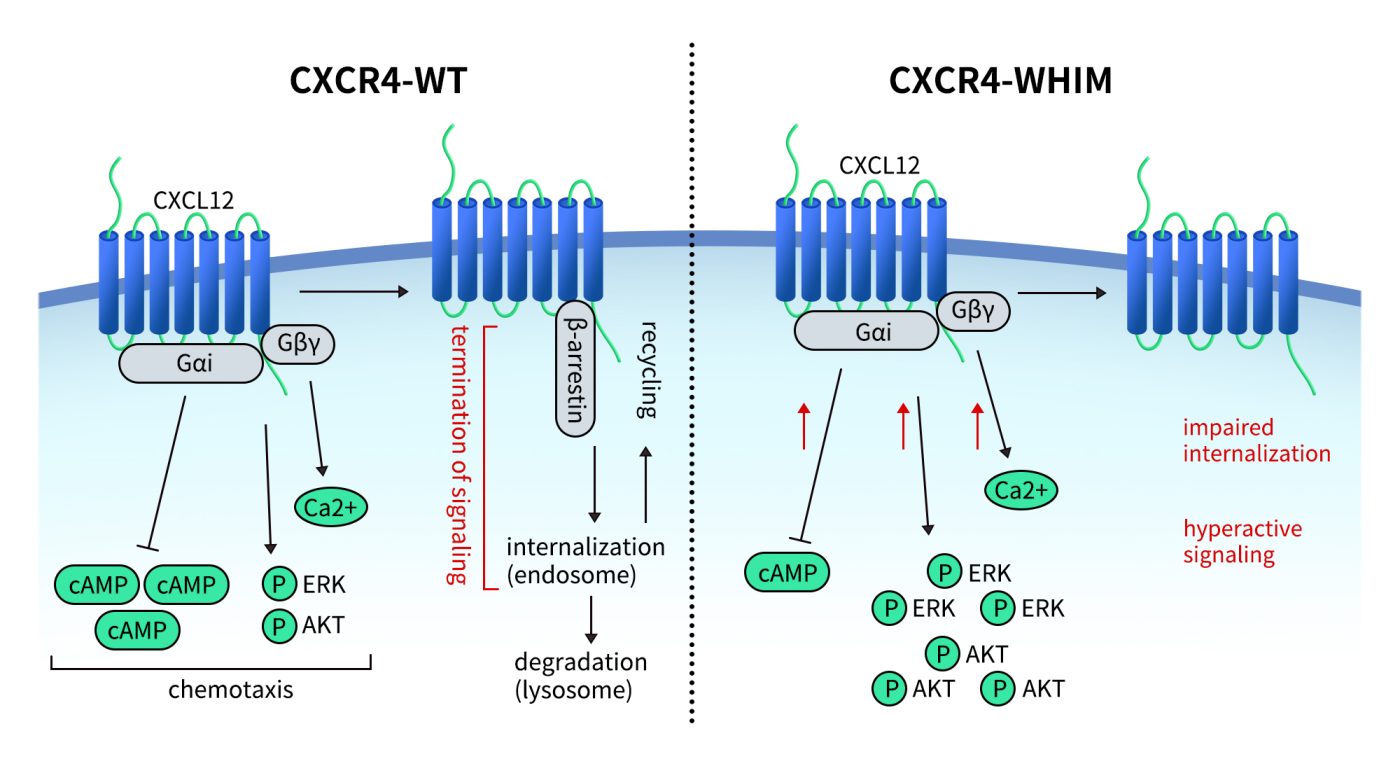
Fig 3.Schematic representation of signaling pathways activated downstream of the CXCR4 receptor and the effect of CXCR4WHIM mutations.[3]
2.2.2 Autoimmune and Inflammatory Diseases
In conditions such as systemic lupus erythematosus (SLE), rheumatoid arthritis, and inflammatory bowel disease, elevated CXCR4 expression drives the accumulation of inflammatory cells. CXCR4 inhibitors may help reduce inflammatory responses, alleviate tissue damage, and limit the formation of immune lesions.
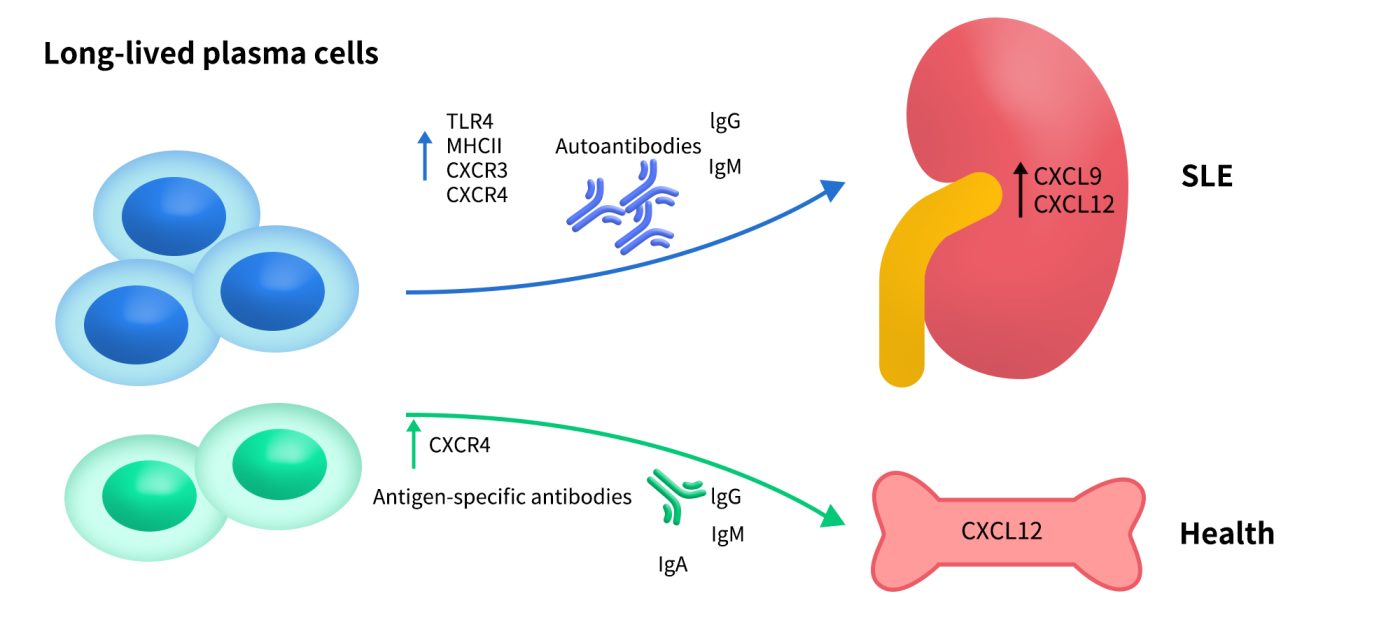
Fig 4. Long-lived plasma cells in SLE. The upregulated CXCL9 and CXCL12 in the inflamed organs of SLE, such as kidney, may recruit the autoreactive long-lived plasma cells to maintain local autoimmune inflammation.[4]
2.3 Stem Cell Therapy and Tissue Repair
The CXCR4–CXCL12 axis is a key regulatory pathway for the homing, retention, and mobilization of hematopoietic stem cells. The FDA has approved Plerixafor and Motixafortide for stem cell mobilization in patients with multiple myeloma.
In addition, CXCR4 regulates the migration and localization of cardiac stem cells and neural progenitor cells, showing potential for post-myocardial infarction repair and neural regeneration after injury.

Fig 5.Pleiotropic roles of Stromal cell-derived factor-1α (SDF-1α)/C-X-C chemokine receptor type 4 (CXCR4) axis in multiple myeloma cells. [5]
2.4 HIV Infection and Viral Entry Mechanism
CXCR4 is one of the two coreceptors—alongside CCR5—used by HIV-1 to enter T cells. X4-tropic HIV strains are more commonly observed in the later stages of AIDS. Although the use of CXCR4 antagonists in HIV treatment remains limited, CXCR4 continues to be a potential auxiliary target for viral suppression.
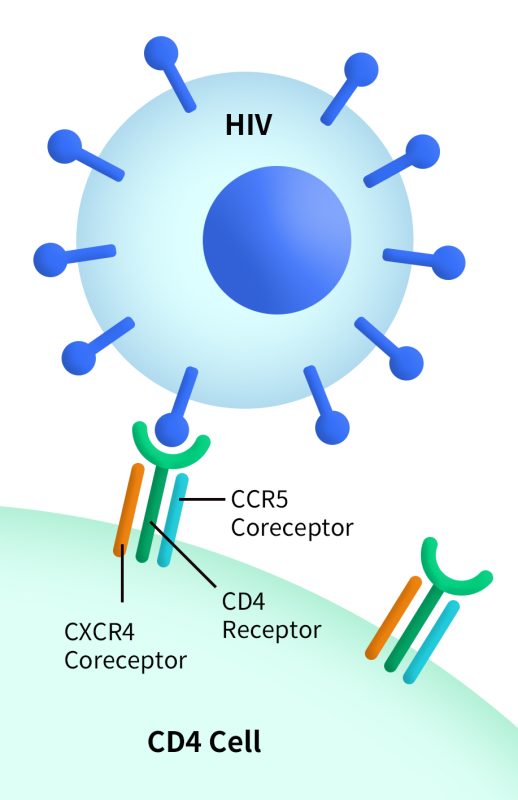
Fig 6.CXCR4 can act as a coreceptor (a second receptor binding site) for HIV when the virus enters a host cell. (https://clinicalinfo.hiv.gov/)
2.5 Radiotracing and Diagnostic Applications
CXCR4-targeted imaging agents, such as 68Ga-Pentixafor, can be used to:
- Visualize CXCR4 expression levels in tumor tissues
- Assess tumor heterogeneity and drug resistance
- Identify patients suitable for CXCR4-targeted therapies
Such theranostic approaches—integrating diagnostics and therapeutics—are becoming an important component of precision oncology.
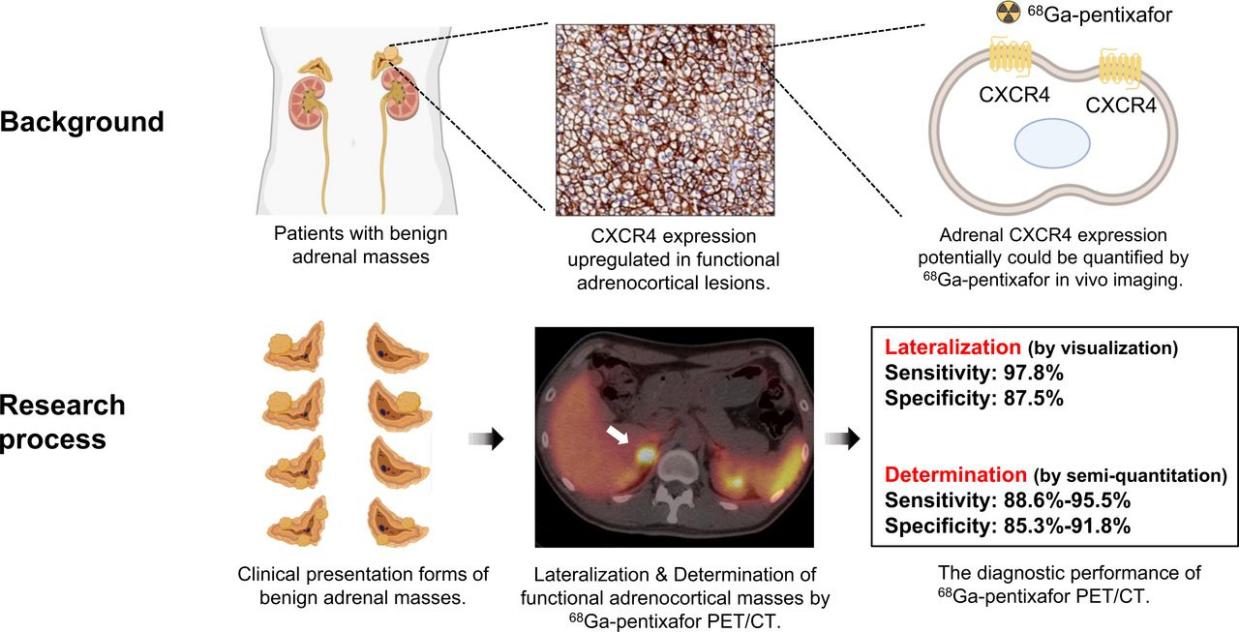
Fig 7.68Ga-pentixafor PET/CT imaging in noncancer patients with suspected adrenal masses. [6]
3. CXCR4-Targeted Drugs
Currently, drug development targeting CXCR4 has advanced into multiple therapeutic areas, including hematopoietic stem cell mobilization, cancer treatment, rare immunodeficiency diseases, and imaging diagnostics. The drug types cover small molecules, peptides, polypeptides, monoclonal antibodies, radioactive probes, and cell and gene therapies. Below is an overview of representative drug developments:
Plerixafor
The earliest approved CXCR4-targeted drug is Plerixafor (AMD3100), developed by Genzyme/Sanofi. It is a small molecule antagonist that competitively blocks the binding of CXCR4 to its ligand CXCL12, promoting the release of hematopoietic stem cells from bone marrow into peripheral blood. In 2008, Plerixafor was approved by the FDA for hematopoietic stem cell mobilization in patients with multiple myeloma and non-Hodgkin lymphoma, making it the first marketed product in the CXCR4 field.
Motixafortide
Following Plerixafor, Motixafortide (BL-8040) became the second clinically approved CXCR4 antagonist. It is a high-affinity peptide-like small molecule developed by BioLineRx in Israel and received FDA approval in 2023 for use in combination with G-CSF to mobilize hematopoietic stem/progenitor cells (HSPCs) in multiple myeloma patients. Motixafortide has a long-acting effect that can improve stem cell collection efficiency and is also being explored in exploratory studies for various solid tumors (e.g., pancreatic cancer) and combination immunotherapy regimens.
Mavorixafor
Another breakthrough drug is Mavorixafor (X4P-001), developed by X4 Pharmaceuticals. It is a small molecule antagonist designed to target WHIM syndrome, a rare immunodeficiency caused by CXCR4 gene mutations. In WHIM patients, CXCR4 fails to undergo normal internalization, resulting in sustained signaling activation. Mavorixafor modulates CXCR4 activity and improves immune cell distribution. It was approved by the FDA in April 2024, marking the first successful clinical application of CXCR4 targeting in genetic immune diseases.
Ulocuplumab
In the antibody category, Ulocuplumab (BMS-936564) is a CXCR4 monoclonal antibody developed by Bristol-Myers Squibb. It blocks ligand binding and induces antibody-dependent cellular cytotoxicity (ADCC). Currently, it is in clinical phase I/II trials for hematological malignancies such as acute myeloid leukemia (AML) and chronic lymphocytic leukemia (CLL).
LY2510924
LY2510924, developed by Eli Lilly, is a peptide antagonist under investigation in combination therapies for multiple solid tumors, including non-small cell lung cancer (NSCLC), pancreatic cancer, and head and neck cancer. This drug blocks tumor cell migration via the CXCR4-CXCL12 axis and enhances T cell infiltration into tumors.
68Ga-Pentixafor
For molecular imaging and precision diagnostics, 68Ga-Pentixafor is a radiolabeled small molecule CXCR4 ligand widely used in PET/CT imaging to identify tumors with high CXCR4 expression (such as lymphoma, prostate cancer, pancreatic cancer, etc.) and provide molecular imaging support for subsequent targeted therapies. Such radioactive CXCR4 imaging agents are promoting the integration of diagnosis and therapy (theranostics).
CXCR4-CAR-T
Additionally, CXCR4 is used to enhance CAR-T cell therapies. For example, CXCR4-CAR-T programs developed by companies such as China’s Keji Pharma engineer T cells to express CXCR4, improving their ability to penetrate tumor stroma and enhance tumor infiltration, particularly in solid tumors like pancreatic and gastric cancers. These therapies are currently in preclinical or early clinical exploratory stages.
POL6326
POL6326 (Balixafortide), developed by Swiss company Polyphor (now Spexis Ltd), once attracted attention. It entered clinical phase II for breast cancer treatment combined with vinorelbine for metastatic breast cancer but was discontinued later due to efficacy and competitive reasons.
NOX-A12
NOX-A12 (Olaptesed pegol) is a Spiegelmer® nucleic acid drug developed by NOXXON Pharma, targeting CXCL12 instead of CXCR4 directly. By neutralizing CXCL12, it blocks downstream CXCR4 signaling. It is currently under phase I/II clinical trials combined with radiotherapy for indications such as pancreatic cancer and glioma.
In summary, CXCR4-targeted drug development has evolved into a diversified landscape ranging from traditional small molecules to antibodies, peptides, radioactive probes, and cell therapies. Its clinical applications have expanded from hematopoietic stem cell mobilization to tumor treatment, genetic immune diseases, precision diagnostics, and enhancement of cell therapies. With deeper understanding of the target and expanding technology platforms, CXCR4’s strategic role in future precision medicine systems will continue to grow.
4. Dima’s CXCR4-Related Products
Dima currently offers a range of CXCR4-targeted off-the-shelf products, including recombinant proteins, full-length membrane proteins, recombinant monoclonal antibodies, and biosimilar reference antibodies. Additionally, we provide comprehensive services such as protein/antibody custom development, antibody humanization, affinity maturation, and stable cell line generation. Moreover, we have established a CXCR4-specific B cell seed library that can screen lead antibody molecules tailored to client needs within just 20 days.
- Recombinant Protein
Human CXCR4 Protein, hFc Tag (Cat.No.PME100834)
- Full-length Membrane Proteins
Human CXCR4 full length protein-synthetic nanodisc (Cat.No.FLP100074)
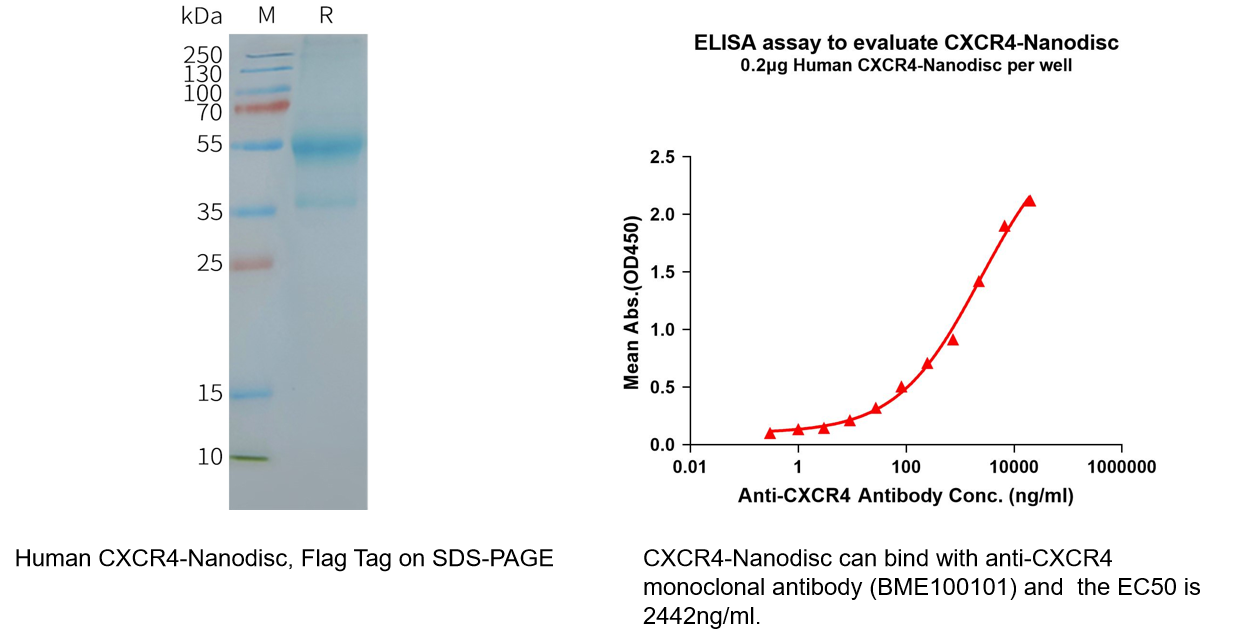
Biotinylated Human CXCR4 full length protein-synthetic nanodisc (Cat.No.FLP100074B)
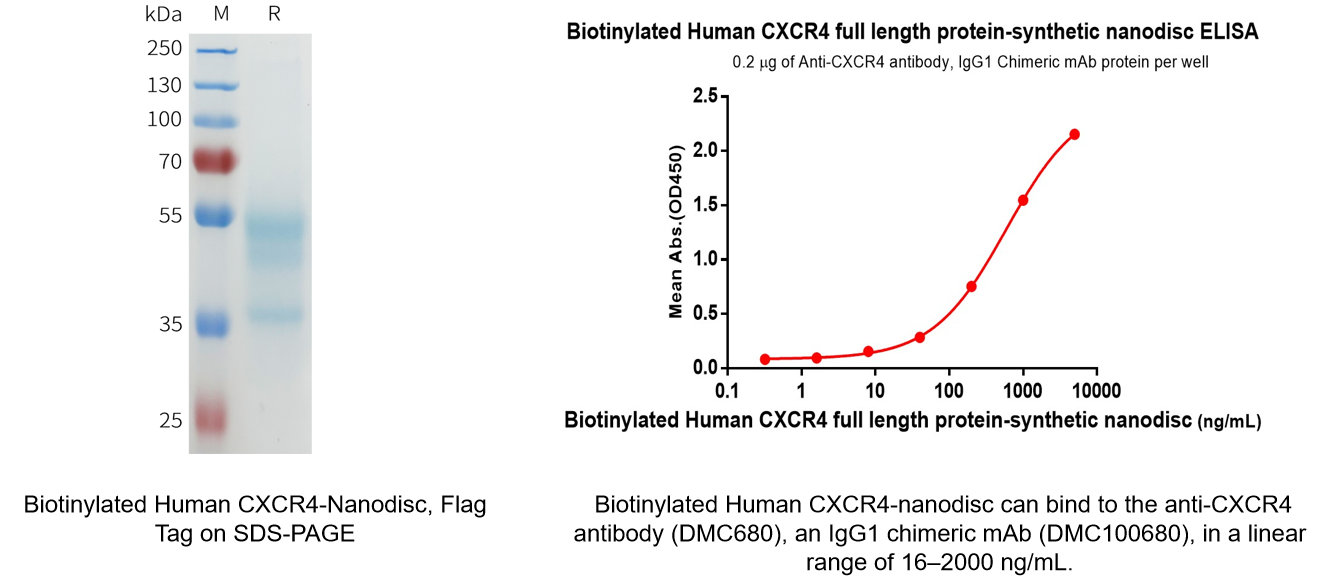
- Monoclonal Antibody
Anti-CXCR4 antibody(DMC680), IgG1 Chimeric mAb (Cat.No.DMC100680)
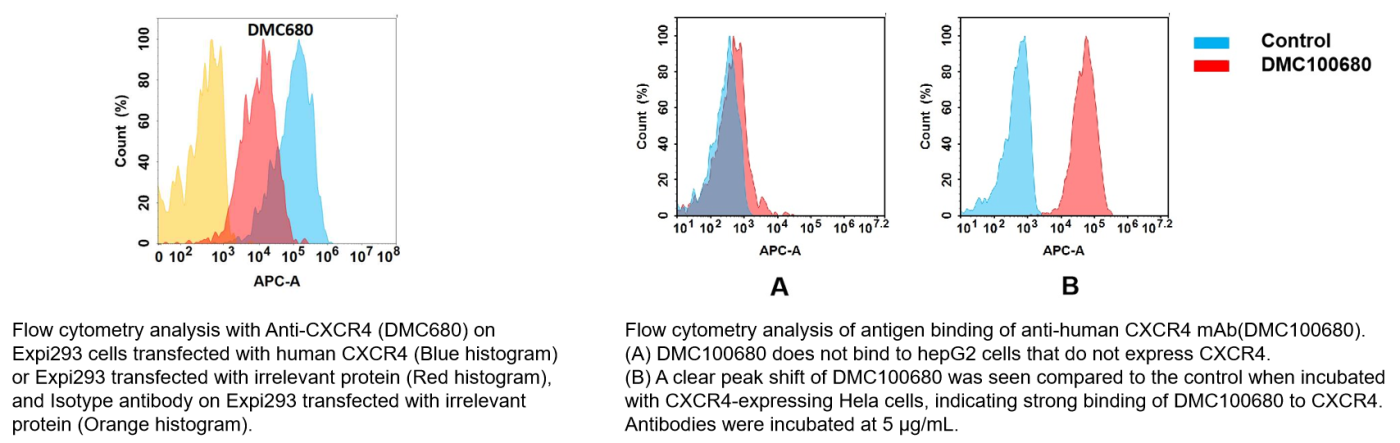
- Biosimilar Reference Antibody
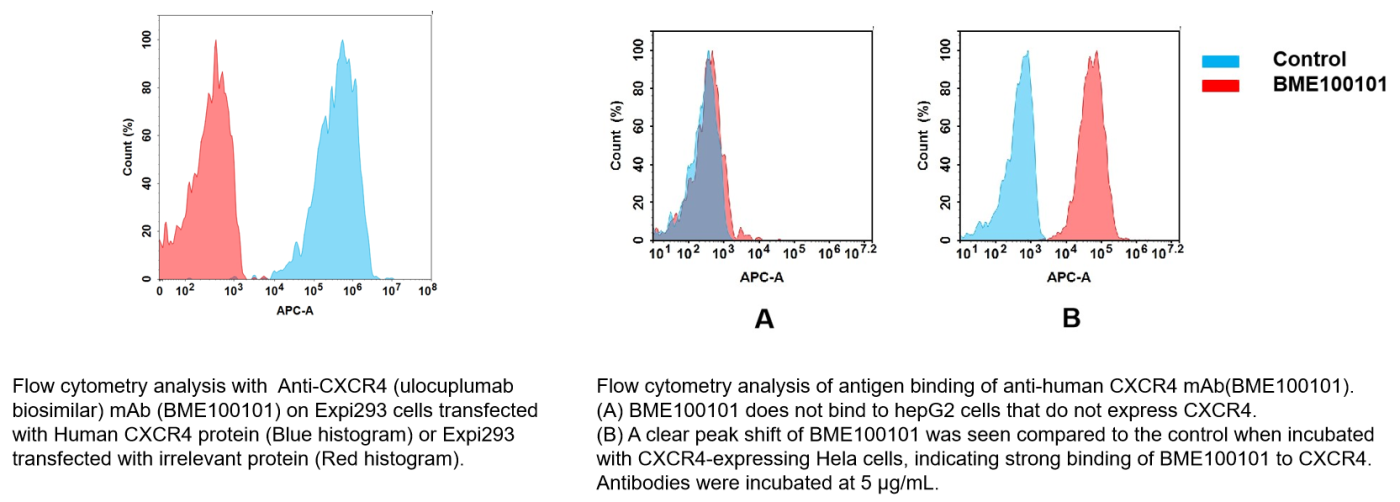
Anti-CXCR4(ulocuplumab biosimilar) mAb (Cat.No.BME100101)
- CXCR4 target-related products
| Type | SKU | Product Name |
| ECD Proteins | PME100834 | Human CXCR4 Protein, hFc Tag |
| Full Length Transmembrane Proteins | FLP120074 | Human CXCR4-Strep full length protein-synthetic nanodisc |
| FLP100074 | Human CXCR4 full length protein-synthetic nanodisc | |
| FLP100074B | Biotinylated Human CXCR4 full length protein-synthetic nanodisc | |
| Monoclonal Antibodies | DMC100680 | Anti-CXCR4 antibody(DMC680), IgG1 Chimeric mAb |
| Biosimilar reference antibodies | BME100101 | Anti-CXCR4(ulocuplumab biosimilar) mAb |
| Biotinylated mAb | DMC100680B | Biotinylated Anti-CXCR4 antibody(DMC680), IgG1 Chimeric mAb |
| BME100101B | Biotinylated Anti-CXCR4(ulocuplumab biosimilar) mAb | |
| PE-conjugated mAb | DMC100680P | PE-conjugated Anti-CXCR4 antibody(DMC680), IgG1 Chimeric mAb |
| BME100101P | PE-conjugated Anti-CXCR4(ulocuplumab biosimilar) mAb |
- Progress in CXCR4 Lead Molecule Discovery

References:
- Ge, B., J. Lao, J. Li, Y. Chen, Y. Song and F. Huang (2017). “Single-molecule imaging reveals dimerization/oligomerization of CXCR4 on plasma membrane closely related to its function.” Sci Rep 7(1): 16873.
- RUEDA, A., SERNA, N., MANGUES, R., VILLAVERDE, A. & UNZUETA, U. 2025. Targeting the chemokine receptor CXCR4 for cancer therapies. Biomark Res, 13, 68.
- ZMAJKOVICOVA, K., PAWAR, S., MAIER-MUNSA, S., MAIERHOFER, B., WIEST, I., SKERLJ, R., TAVERAS, A. G. & BADARAU, A. 2022. Genotype-phenotype correlations in WHIM syndrome: a systematic characterization of CXCR4(WHIM) variants. Genes Immun, 23, 196-204.
- MA, K., DU, W., WANG, X., YUAN, S., CAI, X., LIU, D., LI, J. & LU, L. 2019. Multiple Functions of B Cells in the Pathogenesis of Systemic Lupus Erythematosus. Int J Mol Sci, 20.
- ITO, S., SATO, T. & MAETA, T. 2021. Role and Therapeutic Targeting of SDF-1alpha/CXCR4 Axis in Multiple Myeloma. Cancers (Basel), 13.
- Ding, J., et al., Functional Characterization of Adrenocortical Masses in Nononcologic Patients Using (68)Ga-Pentixafor. J Nucl Med, 2022. 63(3): p. 368-375.