Off-the-shelf Lead mAbs, Ready to Out-Licensing
To help pharmaceutical companies accelerate the preclinical development of antibody drugs, DIMA BIOTECH (hereinafter referred to as “DIMA”) has launched the “All Druggable Targets” lead antibody molecule development program, leveraging its proprietary, innovative single B cell development platform. DIMA will generate lead antibody molecules and corresponding B cell seed libraries targeting all druggable targets. Clients can directly import lead antibody molecules from DIMA or rapidly screen for additional lead antibodies from the B cell seed library. Compared to traditional hybridoma technology, DIMA can save clients up to nearly a year in development time.
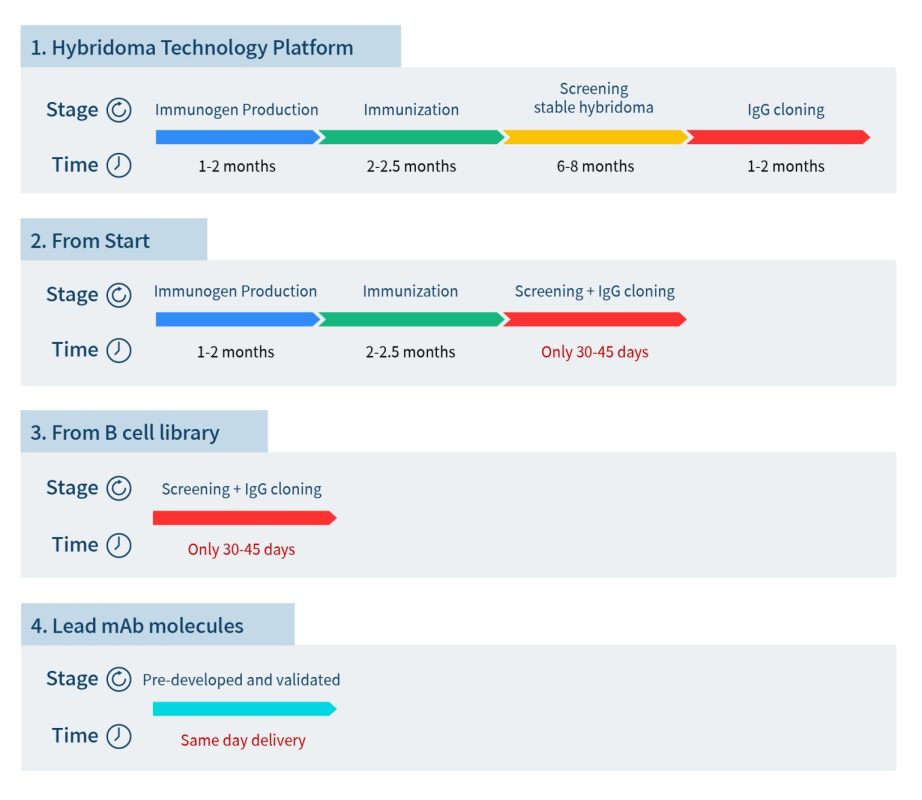
To date, DIMA has screened over 5,000 lead antibody sequences, covering over 500 druggable targets. All of these have been sequenced and are available for global licensing. For some popular target sequences, DIMA has also conducted human-monkey cross-talk and in vitro functional validation. Clients can immediately import sequences and data packages for functional validation, enabling them to be one step ahead and seize market opportunities.
DIMA's Features
Off-the-Shelf Lead mAbs

- No Upfront: Pay after testing
- No Waiting: Same-day delivery
- No risk: No payment if validation is invalid
- High quality: All antibodies are FC-validated
- High efficiency: Save at least 8 months of R&D time
B Cell Seed library
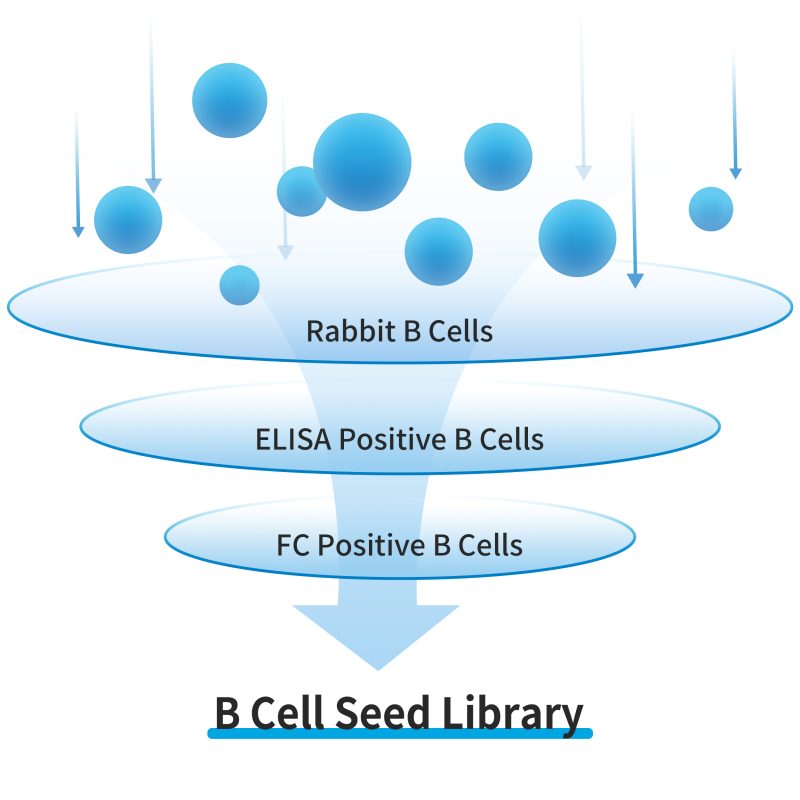
- No risk: No payment if validation is invalid
- Diversity: Multiple FACS binders
- High quality: Serum-free polyclonal Ab identification and FACS functional validation
- High efficiency: Save at least 6 months of R&D time
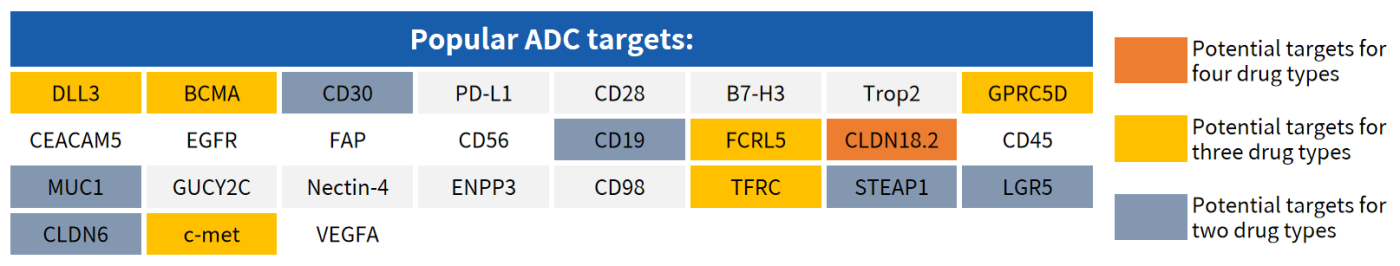
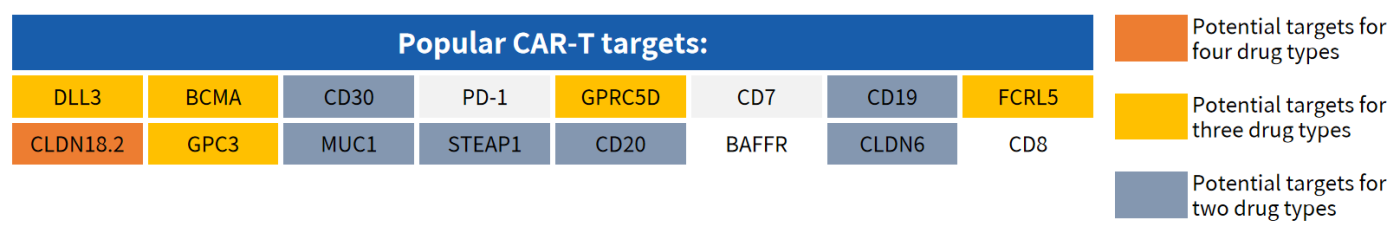
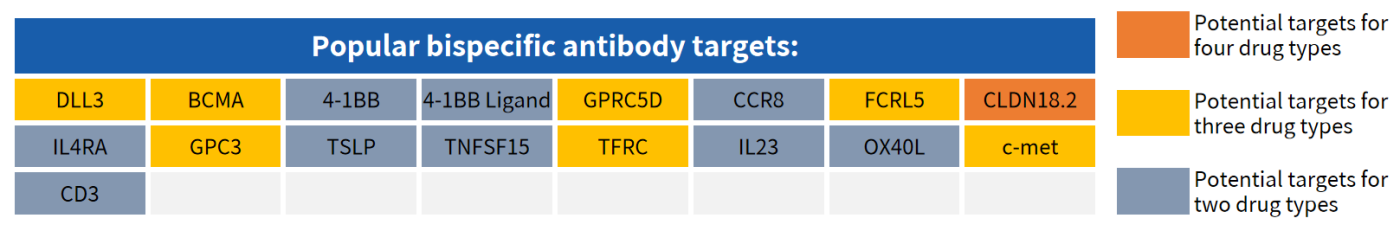
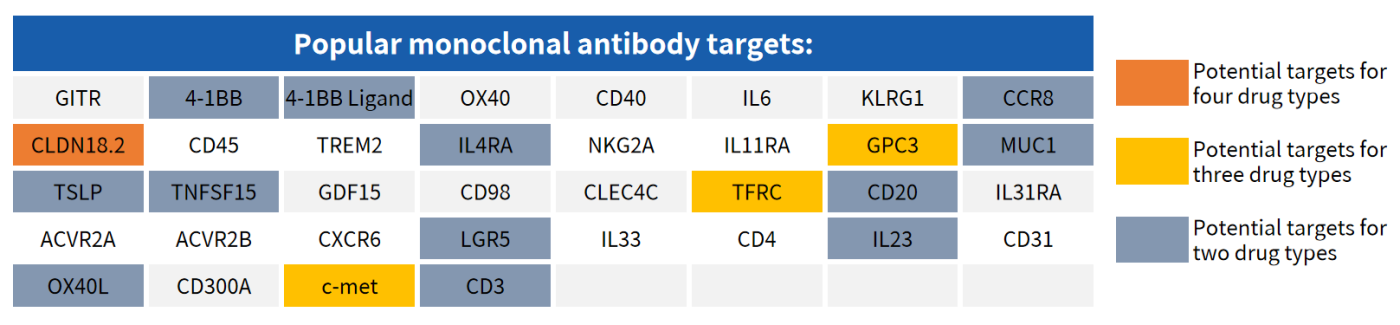
Hot Targets

Case Study
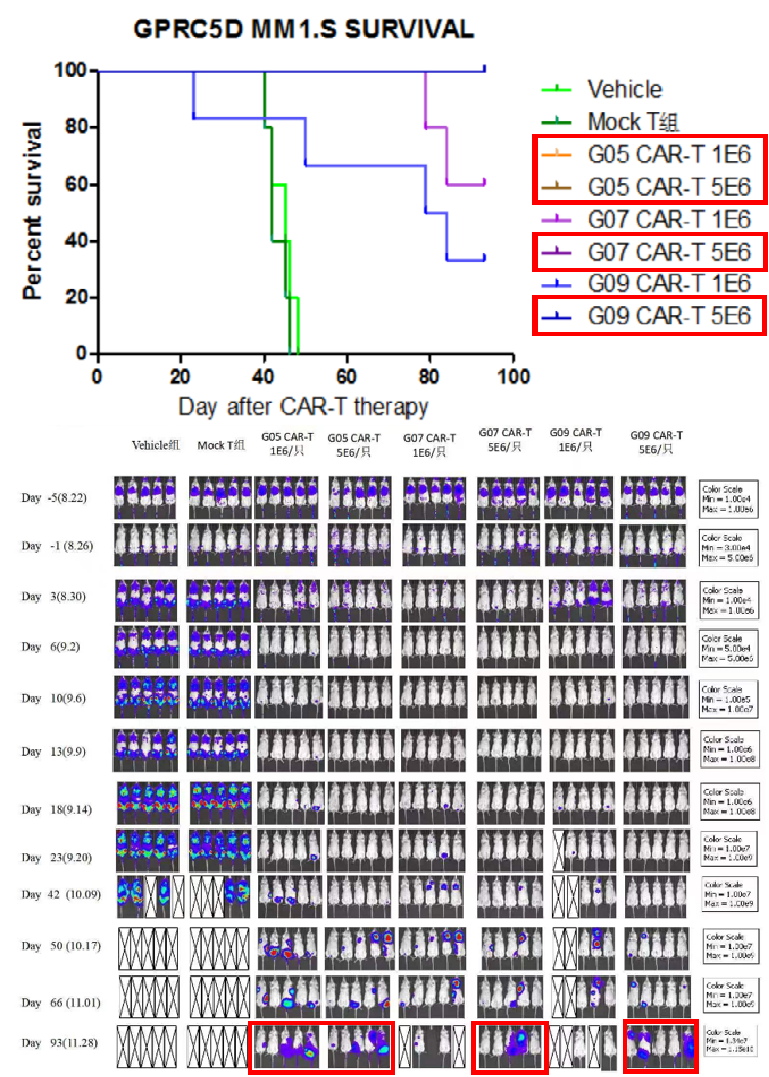
Animal survival data summary on selected CAR constructs
93 days post CAR T-cells infusion, red box-labelled groups of mice are still alive.
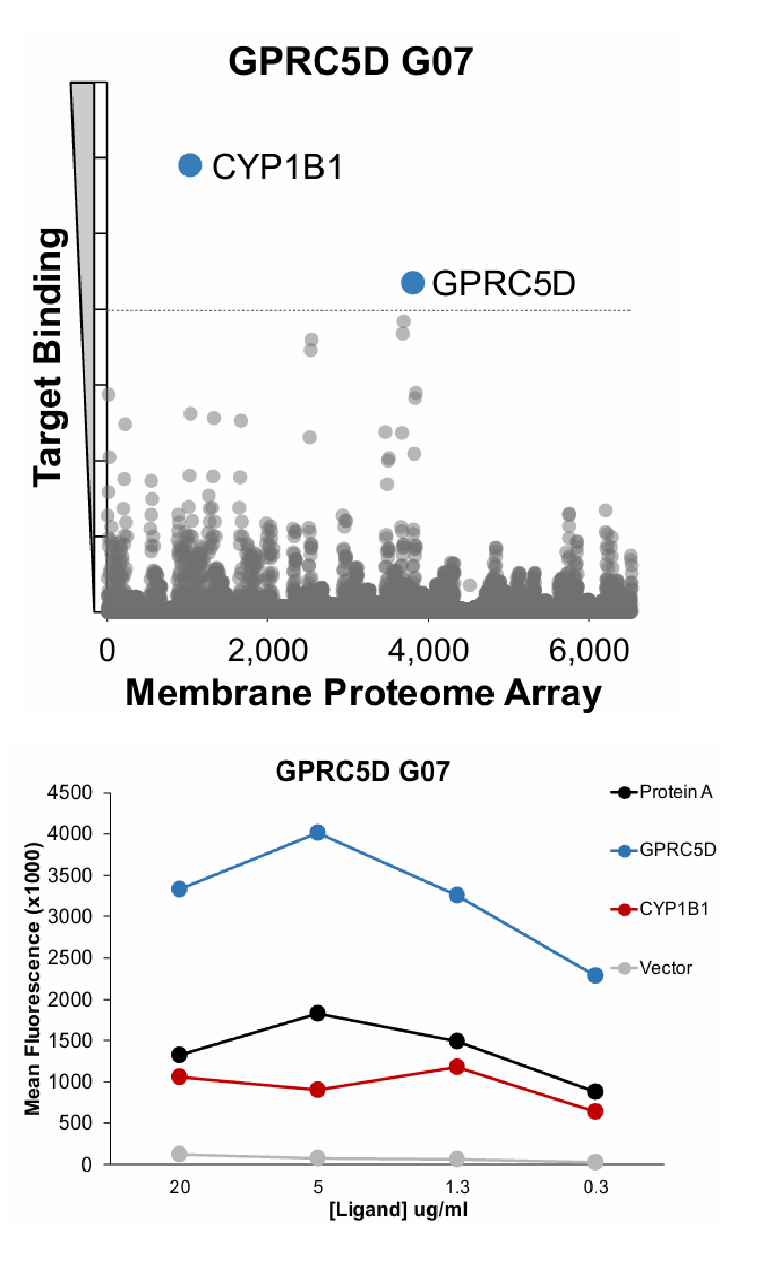
GPRC5D G07 membrane protein array (MPA)
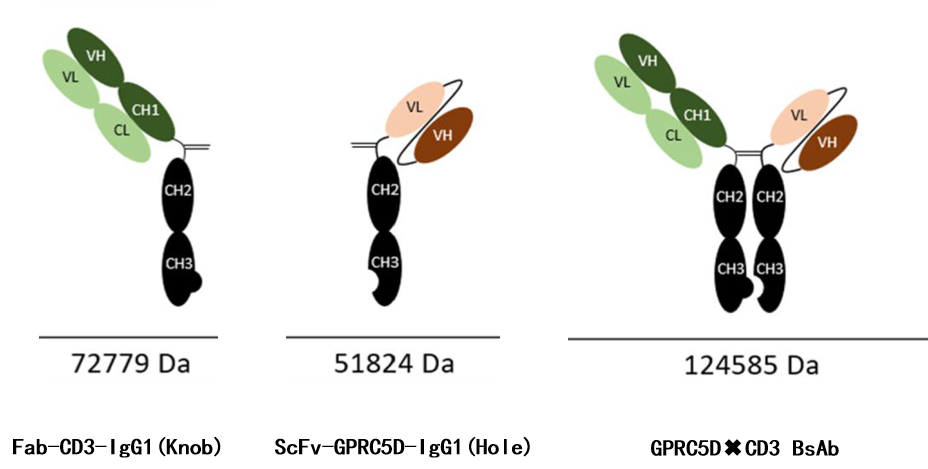
DIMA’s GPRC5D x CD3 BsAb format (KIH)
DIMA utilizes Fab-ScFV-IgG1(KIH) format CD3 :SP34
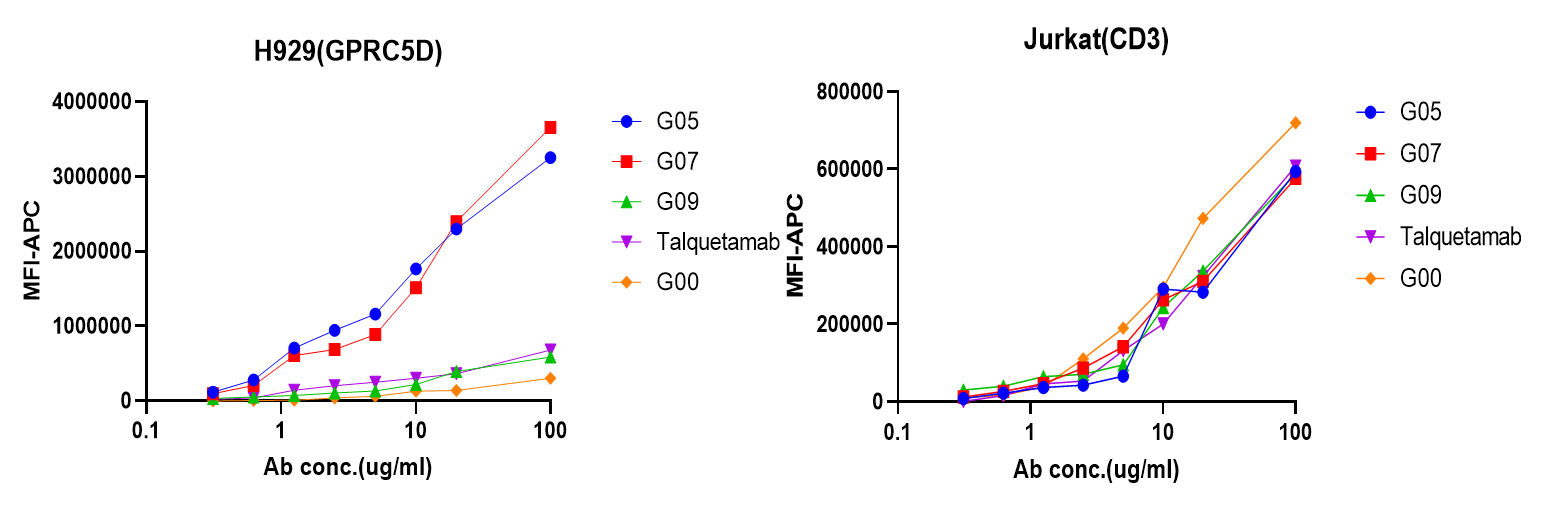
BsAb(GPRC5D X CD3) affinity ranking by flow assay
G05, 07 and 09 are DIMA’s BsAb; Talquetamab is J&J’s BsAb; G00 is the BsAb derived from scFv of Eureka and BMS’s MCARH109
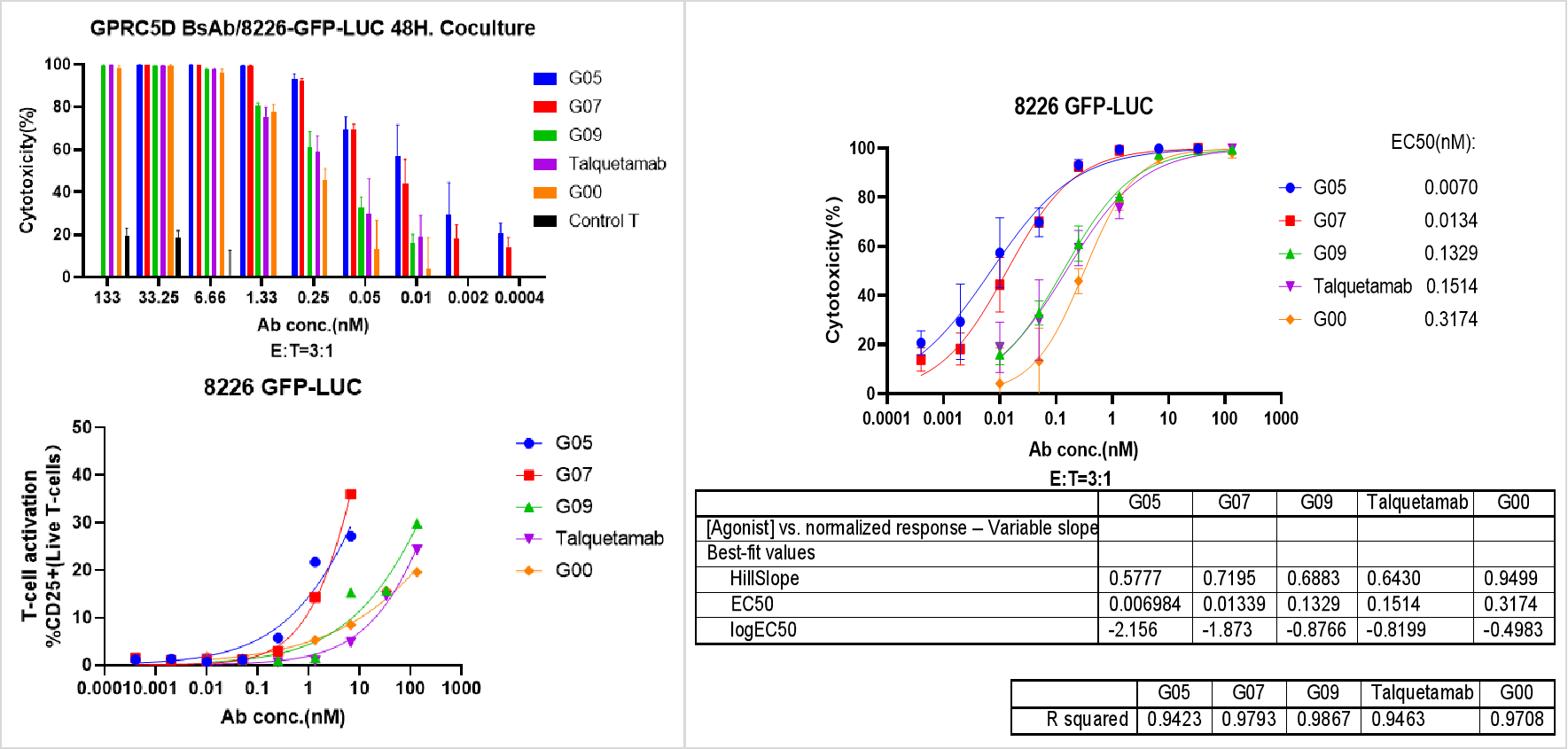
In Vitro tumor cell (8226-GFP-LUC) killing assays with BsAbs
T cells and tumor cells were mixed at 3:1 ratio and different concentration of BsAbs were added and further incubated for 48hrs before assay analysis.
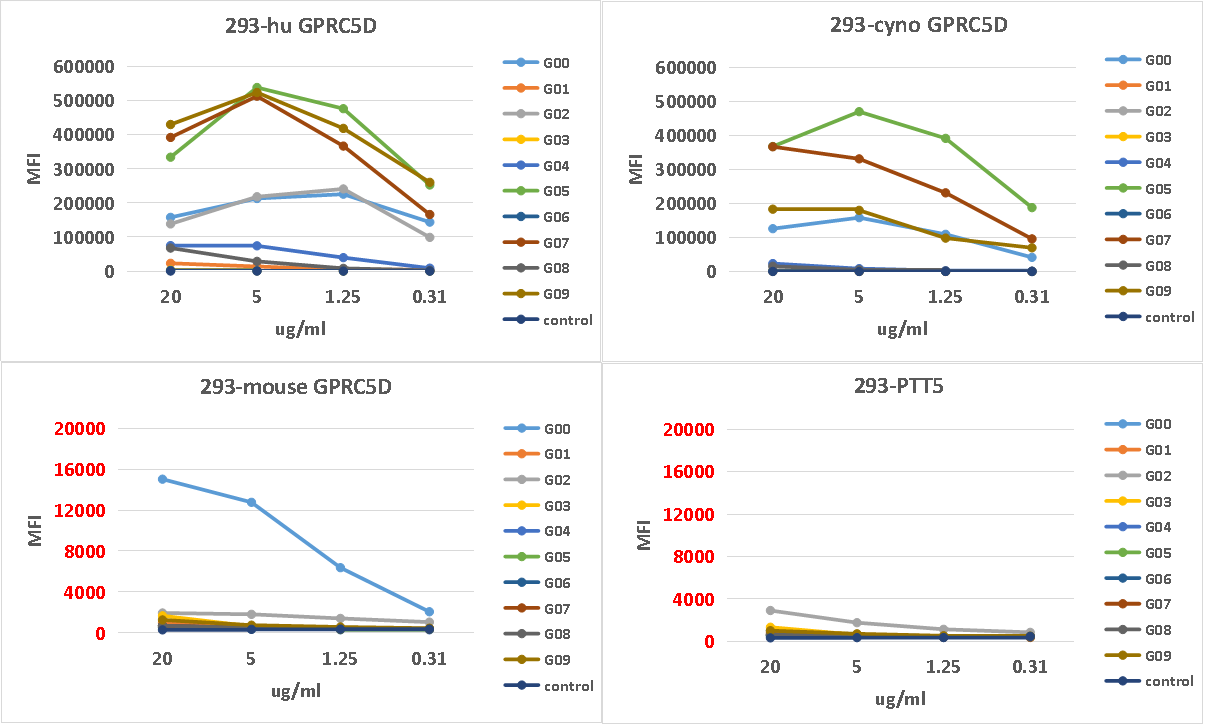
Cyno and mouse cross-reactivity tests
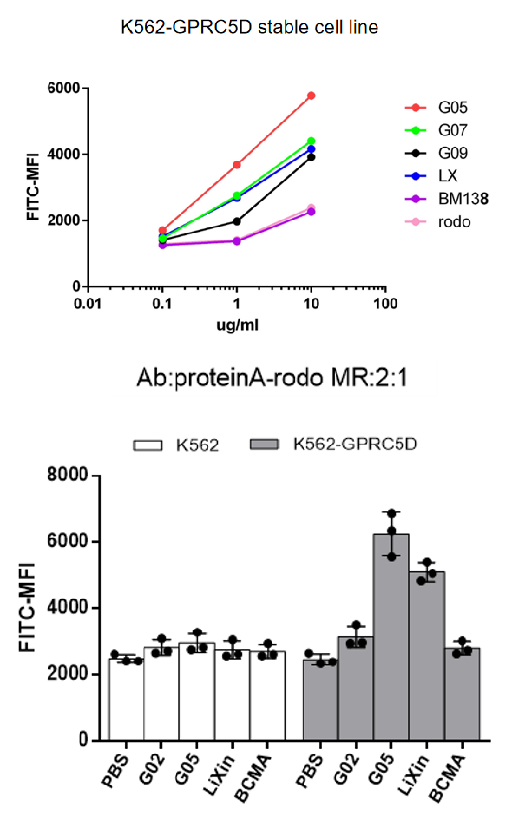
Clone G05 exhibits strong internalization property
Clone G05 exhibits strong internalization property
