DiNabodyTM Nanobody® Discovery Service
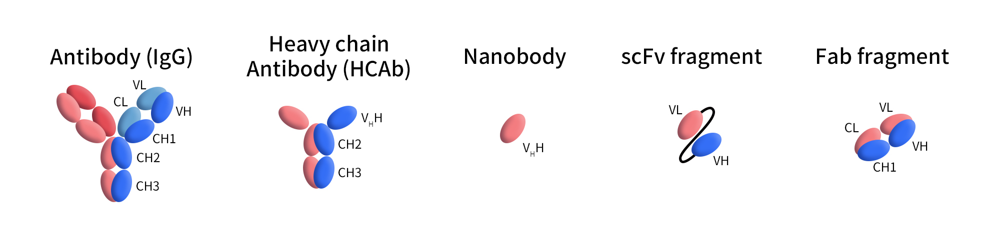
The Concept and advantages of Nanobody®/VHH
In the late 1980s, Dr. Hamers’ team in Belgium discovered that, besides conventional IgG1 antibodies, camel serum contains about 75% antibodies made of only heavy chains without light chains — the Heavy Chain-Only Antibodies (HCAbs). These are also present in llamas and alpacas. The N-terminal domain of HCAbs, capable of directly binding antigens, was later defined as a single-domain antibody (sdAb, VHH, or Nanobody) and has since been widely used in antibody drug development. Comparing to other antibodies, VHH antibodies have several advantages as follows:
- High stability and solubility: Evolution increased hydrophilicity to compensate for the missing light chain, allowing them to remain stable even at 70 °C.
- Low immunogenicity: Despite evolutionary distance, camelid antibodies share higher homology with human antibodies than rodent ones.
- Deep epitope access: Longer CDR3 regions enable binding to recessed antigen sites.
- Easy engineering: Their simple structure facilitates affinity maturation, humanization, and bispecific antibody design.
- Small size: Llama-derived nanobodies (~15 kDa) can penetrate tissues more effectively.
Nanobody® is a registered trademark of Ablynx N.V., a Sanofi company.
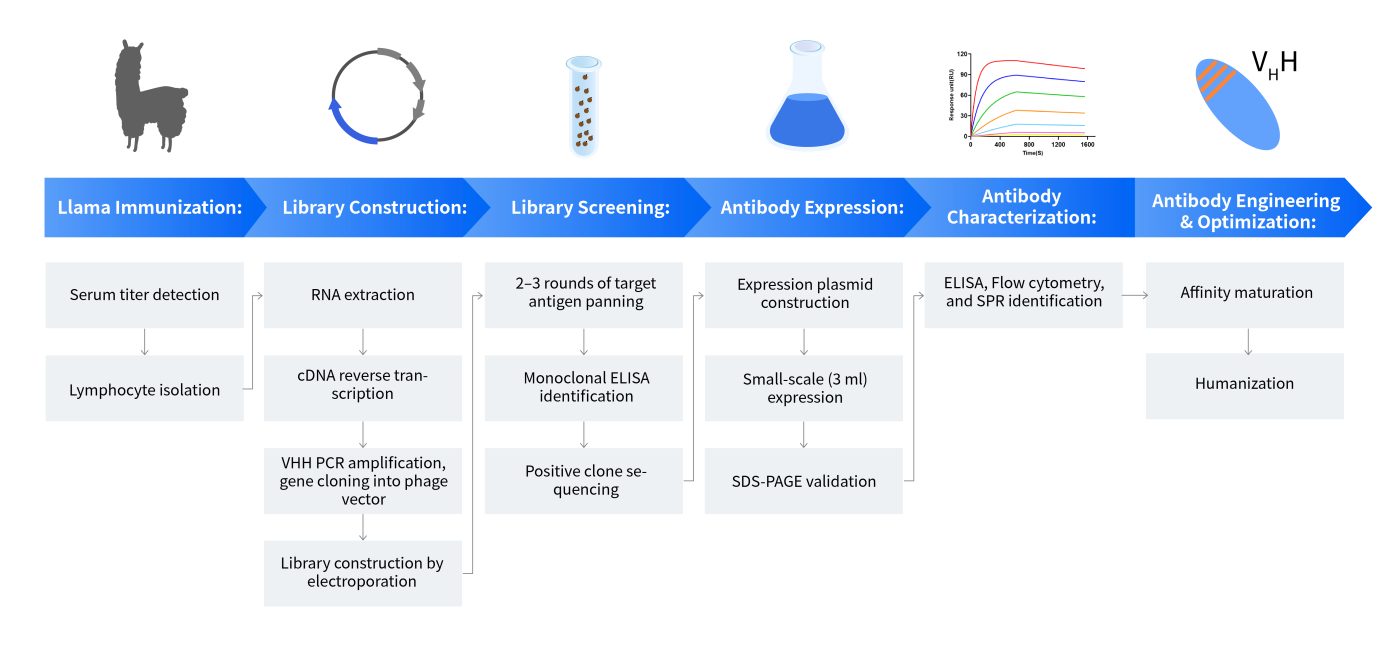
The Workflow of Nanobody®/VHH Discovery
DIMA Features

Efficient delivery of FACS-validated nanobody sequences

Customer-specific llama allocation to avoid repeated immunization

Dual technology platform: phage display and mammalian cell display

One-stop service for humanization and affinity maturation
Nanobody®/VHH Project Timeline and Deliverables
| Stage | Service Content | Time Frame | Deliverables |
|---|---|---|---|
| I | Antigen preparation (or provided by the client) | 2-3 weeks | Quality Control Data |
| II | Animal immunization (Alpacas) | 8-10 weeks | Serum titer report, Serum |
| III | Library construction | 2-3 weeks | library quality assessment report(capacity≥107) |
| IV | Antibody screening(solid-phase, liquid-phase, etc.) | 3-5weeks | ELISA assay report, positive nanobody sequences |
| V | Antibody expression and evaluation (ELISA, FACS, SPR,etc.) | 3 weeks | assessment report |
| VI | Report compilation | 1 weeks | project report |
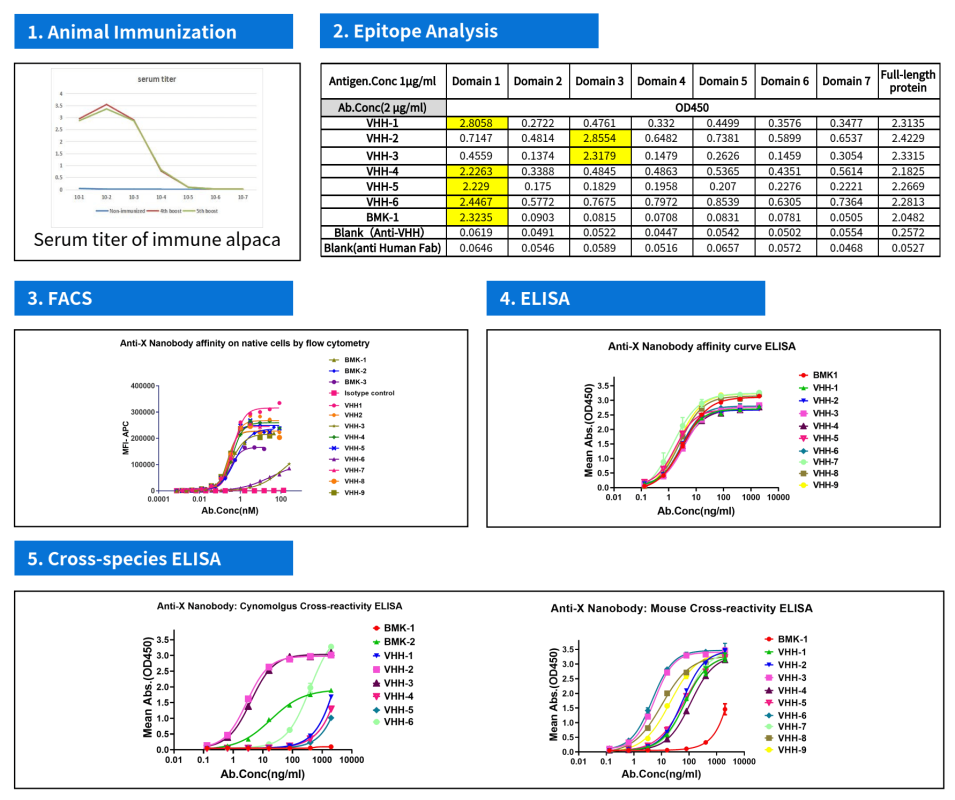
Case Study on the Development of Target X