In the previous article, we focused on the popular drug target for weight loss, G Protein-Coupled Receptor 75 (GPR75). This time, our topic is another emerging target for treating adult obesity, Activin A Receptor Type 2 (ActRII). Unlike currently approved weight loss drugs, which can reduce the weight of obese patients but often lead to muscle loss, targeting ActRII aims to address this issue by preserving skeletal muscle mass while enhancing fat loss. This is particularly important for older individuals, as muscle loss can make them more vulnerable and prone to accidents. What exactly is ActRII? What is its specific mechanism of action? And which pharmaceutical companies are currently investing in this target?
1. The Structure of ActRII
ActRII belongs to the TGF-β receptor family, which regulates the signaling of transforming growth factor β ligands. Currently, there are two types of Activin A Receptor Type 2, ACVR2A and ACVR2B. Among them, ACVR2A (also known as ActRIIA) was first identified as a transmembrane serine/threonine kinase for activin A by Mathews LS et al. in 1991 [1]; ACVR2B (also known as ActRIIB) was discovered by Harrison CA et al. in 2005. ACVR2B plays a crucial role in cellular signaling [2]. The biological function of ActRII is closely intertwined with its counterpart, Activin A Receptor Type I (ActRI), of which seven types have been identified, including ALK1, ALK2, ALK3, ALK4, ALK5, ALK6, and ALK7 [3]. Both types of Activin receptors have approximately 500 amino acid residues, are single-pass transmembrane serine/threonine kinases, and consist of an extracellular domain rich in Cys residues (approximately 110 amino acid residues) with ligand-binding activity, a transmembrane domain (approximately 26 amino acid residues), and an intracellular domain (approximately 360 amino acid residues) into the cells.
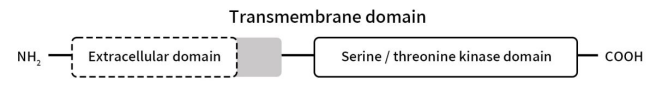
Figure 1. The structure of Activin A Receptors
Unlike type II receptors, type I receptors possess a unique GS domain near the membrane-proximal region in the cytoplasm, preceding the kinase domain. In the ligand-binding complex, type II receptors phosphorylate serine and threonine residues within the GS region of type I receptors [4]. This phosphorylation event transforms the GS region from a docking site for the inhibitor protein FKBP12 to a docking site for its substrate—the Smad family transcription factors [5] . ActRII is intricately involved in a myriad of physiological processes, including growth, cell differentiation, homeostasis, Osteogenesis, apoptosis, and many other essential functions.
2. ActRII Signaling Pathway
As shown in the diagram below, activins, growth differentiation factors (GDFs) such as Myostatin (GDF8), and GDF11 initiate signal transduction through ActRII and ActRI receptors. ActRII is the primary ligand-binding receptor, which binds to ligands with high affinity to form ligand-receptor complexes. The ligand/ActRII complex further recruits and binds to ActRI. Upon binding, ActRI is phosphorylated and activated by ActRII. Smad2 and Smad3 are receptor-regulated Smads specific to activin/TGF-β signaling and are phosphorylated by activated ActRI. In the nucleus, the Smad2/3/4 complex regulates gene expression by interacting with other transcriptional co-factors. Additionally, Smad-independent pathways (such as MAPK) are activated downstream of activin receptors. Inhibitors counteract activins by forming high-affinity complexes with ActRII and β-glycans. Follicle-stimulating hormone, Myostatin propeptide, and receptor extracellular domain inhibit the activity of activins and related factors in the extracellular space to prevent ligand-receptor interactions.
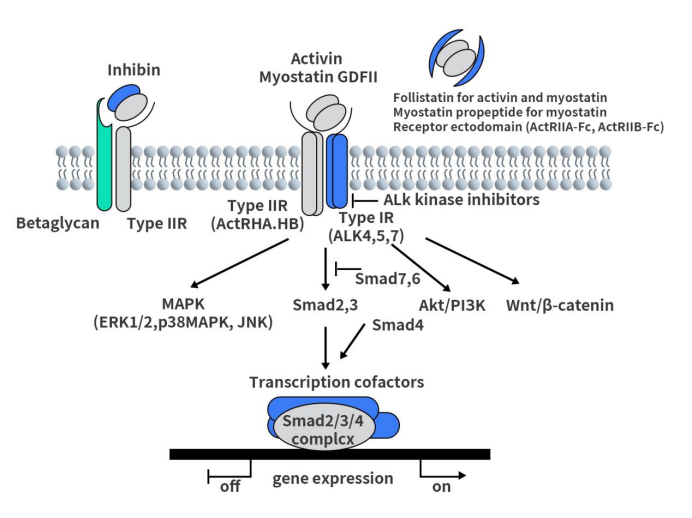
Figure 2. ActRII signaling pathway
The signaling triggered by the binding of activins to ActRII serves as the executor of vital physiological activities such as embryonic development, organ formation, hormone synthesis, cell differentiation, and immune regulation. Abnormalities in this pathway are highly associated with various diseases, including metabolic and immune disorders such as skeletal muscle atrophy, obesity, anemia, and pulmonary fibrosis. For instance, in obesity and heart failure, the activin pathway can directly promote lipid storage and myocardial cell protein degradation, ultimately leading to visceral fat accumulation and impaired myocardial contraction. GDF8, a member of the TGF-β superfamily, is a negative regulator of muscle growth. Myostatin binds to ACVR2B and, to a lesser extent, to ACVR2A. The GDF8/ActRII pathway has been identified as a key regulator of skeletal muscle size.
3. Clinical Research Progress on ActRII Targets
Currently, there are two ActRII protein products available in commercial production on the global market, with six ActRII products entering the clinical stage, including four proteins and two antibodies.
- Sotatercept
Sotatercept, developed by Acceleron, is a “first-in-class” ActRIIA-Fc fusion protein. In 2021, the drug received FDA breakthrough therapy designation. In October 2021, Merck acquired Acceleron for $11.5 billion, gaining ownership of Sotatercept. Sotatercept combines the modified extracellular domain of ActRIIA with the Fc region of an antibody. It can block the binding of activin to receptors on the cell membrane, thereby reducing activin-mediated signaling. Sotatercept was approved by the FDA for marketing on March 26, 2024, for the treatment of patients with pulmonary arterial hypertension (PAH).
- Luspatercept
Luspatercept, developed by BMS, is an ActRIIB-Fc fusion protein composed of the Fc domain of human immunoglobulin G1 (IgG1) fused with the extracellular domain of activin receptor IIB (ActRIIB). In July and August 2014, Luspatercept received orphan drug designation for the treatment of β-thalassemia intermediate and severe and myelodysplastic syndromes (MDS), respectively. In November 2019, Luspatercept was first approved by the FDA for the treatment of adult patients with β-thalassemia who require regular red blood cell (RBC) transfusions. In April of the following year, Luspatercept was approved by the FDA for the treatment of anemia associated with low-risk MDS in adult patients.
- Bimagrumab
Bimagrumab, initially developed by Novartis and MorphoSys, is a monoclonal antibody targeting activin type IIA/B receptors (ActRIIA/B), which blocks the ActRIIA/B signaling pathway by binding to ActRIIA/B, showing potential in the treatment of muscle wasting and obesity. Clinical trials conducted in 2016 for inclusion body myositis (NCT01925209, NCT02573467, NCT01669174) indicated that bimagrumab did not significantly improve patients’ 6-minute walk distance (6MWD), muscle strength, and grip strength; it only increased muscle mass, casting doubt on its efficacy.
In February 2017, a phase II clinical trial (NCT03005288) of bimagrumab for obese and type II diabetes patients reignited optimism for its commercial prospects. This development laid the foundation for Lilly’s acquisition of Versanis Bio in 2023, as Lilly explicitly stated that the primary purpose of this acquisition was Versanis’s lead pipeline bimagrumab. Currently, the drug is primarily being developed for obesity indications in clinical settings.
- KER-012
KER-012 is a protein therapeutic candidate developed by the U.S. biotechnology company Keros Therapeutics. It consists of a modified ligand-binding domain of ActRIIB fused with the Fc region of human antibodies. KER-012 is designed to bind to and inhibit the signaling of TGF-β ligands, including activin A and activin B, potentially increasing bone mass. Additionally, inhibition of activin A and activin B by KER-012 also has the potential to increase the BMP signaling pathway. Currently, Keros is conducting a study (NCT05975905) to explore the safety and efficacy of KER-012 in combination with background therapy for the treatment of adult pulmonary arterial hypertension (PAH). This clinical trial is a phase II study and is currently recruiting participants for PAH indications.
- KER-050
KER-050 is an engineered ligand trap developed by Keros Therapeutics, consisting of a modified ligand-binding domain of ActRIIA fused with a partial Fc region of human antibodies. This fusion protein is designed to promote hematopoiesis by inhibiting the signaling of certain members of the TGF-β family of proteins, thereby increasing the production of red blood cells and platelets. Currently, Keros is conducting two phase II clinical studies (NCT04419649 and NCT05037760) for KER-050. The indications for these studies are myelodysplastic syndromes & cytopenias and bone marrow fibrosis, respectively. Both clinical trials are currently recruiting participants.
- LAE102
LAE102 is a monoclonal antibody targeting ActRIIA developed by Laekna Therapeutics, with regulatory effects on tumor growth, immune activation, and hematopoietic development. Laekna initially positioned LAE102 as one of the core pipelines in the field of cancer treatment. Clinical trials for LAE102 in tumors were approved by the FDA and CDE in May 2023 and February 2024, respectively. On March 11th, Laekna announced the submission of a clinical trial application for LAE102 in the indication of weight loss to the FDA, completing dual submissions in the United States and China. This news led to a doubling of Laekna’s stock price.
4. DIMA Biotech: Advancing ActRII Biotherapy Development
DIMA Biotechnology LTD is a biotechnology company specializing in the preclinical development of drug targets and related services. For ActRII, DIMA has developed recombinant human ACVR2A and ACVR2B protein products, which are expressed using mammalian expression systems to preserve protein functionality. Custom production of proteins from various species and related antibody services are also available upon request. In addition, DIMA operates several key technology platforms to facilitate the discovery of ActRII-related therapies:
- DiMPro™ Functional Membrane Protein Development Platform (including ECD, Synthetic Nanodisc, VLP)
- DIMA mAbs Single B Cell Lead Antibody Discovery Platform (400+ drug targets and 5000+ rabbit monoclonal antibody molecules developed within 3 years)
- DiLibrary™ Antibody Engineering Platform (including antibody humanization, antibody affinity maturation, PTM removal, etc.)
- Antibody Functional Validation Platform (including CAR-T, ADC antibody functional evaluation, etc.)
| Target | Cat No. | Product Name |
| ACVR2A | PME101550 | Human ACVR2A Protein, hFc Tag |
| ACVR2B | PME101551 | Human ACVR2B Protein, hFc Tag |
| BME100228 | Anti-ACVR2B(bimagrumab biosimilar) mAb |
Reference:
[1] Mathews LS, Vale WW: Expression cloning of an activin receptor, a predicted transmembrane serine kinase. Cell. 1991, 65: 973-982.
[2] Harrison CA, Gray PC, Vale WW, Robertson DM: Antagonists of activin signaling: mechanisms and potential biological applications. Trends Endocrinol Metab. 2005, 16: 73-78.
[3] Feng XH, Derynck R: Specificity and versatility in tgf-beta signaling through Smads. Annu Rev Cell Dev Biol. 2005, 21: 659-693.
[4] J.L. Wrana, L. Attisano, R. Wieser, F. Ventura, J. Massague, Mechanism of activation of the TGF-beta receptor. Nature, 370, 6488 (1994), 341– 347.
[5] M. Huse, T.W. Muir, L. Xu, Y.G. Chen, J. Kuriyan, J. Massague, The TGF beta receptor activation process: an inhibitor- to substrate-binding switch. Mol. Cell, 8, 3 (2001), 671– 682.
