Since Ganymed unveiled the efficacy data of Zolbetuximab (IMAB362), the anti-Claudin18.2 monoclonal antibody, for the treatment of advanced gastric and gastroesophageal junction adenocarcinoma (GC/GEJ) at the 2016 ASCO conference, Claudin18.2 has emerged as a focal point for pharmaceutical companies eager to advance therapeutic options. These companies explore various technological routes, including monoclonal antibodies, bispecific antibodies, antibody-drug conjugates (ADCs), and CAR-T cells. In October of the same year, Amgen acquired Ganymed for 1.28 billion euros, thereby securing additional commercialization rights to Zolbetuximab. Fast forward to March 26, 2024, when Zolbetuximab once again made headlines as the world’s first approved Claudin18.2 antibody. The Japanese Ministry of Health, Labour and Welfare (MHLW) granted approval for its use in CLDN18.2-positive, unresectable, advanced, or recurrent gastric cancer patients. This milestone undoubtedly invigorates pharmaceutical companies strategically targeting the Claudin18.2 pathway, thrusting Claudin18.2 back into the spotlight. But what exactly is Claudin18.2? And what are the current developments in other therapies aimed at this intriguing target?
1. Claudin18.2 and Claudins
Claudin18.2 (also called CLDN18.2) belongs to the Claudins protein family. The Claudins family consists of integral membrane proteins found in tight junctions of epithelial and endothelial tissues. Claudins are essential components of tight junctions, mechanically connecting adjacent cells to form an epithelial barrier. These tight junctions regulate the movement of ions, water, and other molecules between cells while maintaining cell polarity.
As shown in Figure 1, Claudin proteins are four-pass transmembrane proteins. They are divided into the intracellular and extracellular sides of the cell membrane. The N-terminus and C-terminus of Claudin proteins are both located on the intracellular side of the membrane. The N-terminus is relatively short, consisting of approximately seven amino acids, while the C-terminus contains approximately 25-55 amino acids. The cytoplasmic tail (N-terminus) of Claudin includes several post-translational modification sites, such as phosphorylation, palmitoylation, and SUMOylation. The C-terminus contains a PDZ-binding motif (YV), which allows it to interact with proteins containing PDZ domains. On the extracellular side of the membrane, there are two extracellular loop (ECL) domains: ECL1, which comprises approximately 50 amino acids, and ECL2, which comprises approximately 25 amino acids.
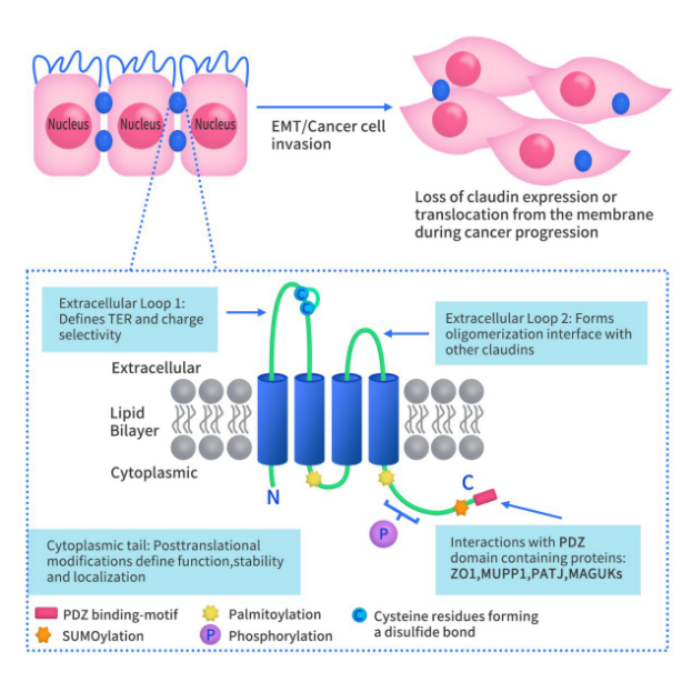
Figure 1. The structure of Claudins [1] [2]
The structure of Claudin proteins enables the Claudins tight protein family to effectively maintain the polarity of epithelial and endothelial cells. Claudin proteins mediate tight junctions through the formation of Claudin protein complexes between different cells. Tight junctions have been confirmed as a major determinant of paracellular permeability. Claudin proteins are primarily expressed in epithelial cells, where their function is to regulate the permeability of barrier structures.
2. The expression of Claudin18.2
Claudin proteins, with 27 members in mammals, play a crucial role in maintaining cell polarity. Among them, Claudin-18 splice variant 2 (CLDN18.2) is a stomach-specific tight junction protein expressed in various cancers. Claudin18.2 shows abnormal activation and overexpression in primary malignant tumors such as breast cancer, colon cancer, liver cancer, head and neck cancer, bronchial cancer, and non-small cell lung cancer. It is particularly prevalent in gastrointestinal malignancies, including gastric cancer (70%), pancreatic cancer (50%), and esophageal cancer (30%). In tumors, Claudin18.2 is involved in cell proliferation, differentiation, and migration.
In normal tissues, cells are tightly adhered, making it extremely challenging for antibody drugs to bind to them. This reduces the impact of Claudin18.2 drugs on normal tissues. However, the development of malignant tumors disrupts tight junctions, exposing Claudin18.2 epitopes on the surface of tumor cells. Consequently, these exposed epitopes can bind to large Claudin18.2 molecule drugs.
The characteristics of Claudin18.2, including its high selectivity and widespread expression only in cancer cells, make it an ideal tumor immunotherapy target. Currently, research targeting the Claudin18.2 pathway primarily focuses on gastric cancer. This discovery brings new hope to the field of cancer treatment and has the potential to improve patient prognosis and survival rates.
3. Advances in Claudin18.2 Targeted Therapy Research
Claudin18.1 is another splice variant of CLDN18. Structurally similar to Claudin18.2, Claudin18.1 differs by only 7 amino acid residues in the extracellular loop 1 (ECL1) sequence. Developing antibodies that specifically recognize Claudin18.2 without cross-reacting with Claudin18.1 remains a challenge. Currently, clinical development of Claudin18.2-targeted therapies encompasses various types, including monoclonal antibodies, bispecific antibodies, ADCs, and CAR-T cell therapy. Among these, Claudin18.2 monoclonal antibodies are the most prominent, followed by ADCs.
3.1 Claudin18.2 Monoclonal Antibodies
Zolbetuximab, also known as GC-182 or iMAB-362, is an IgG1 monoclonal antibody specifically targeting CLDN18.2. Developed by Ganymed, this antibody received approval from the Japanese Ministry of Health, Labour and Welfare (MHLW) on March 27, 2024. It is indicated for the treatment of CLDN18.2-positive, unresectable, advanced, or recurrent gastric cancer patients. Zolbetuximab, marketed under the brand name Vyloy, represents the world’s first approved Claudin18.2-targeted therapy.
The recent approval of Zolbetuximab was primarily based on positive results from the SPOTLIGHT (NCT03504397) and GLOW (NCT03653507) phase III clinical trials. SPOTLIGHT trial aims to evaluate the efficacy of zolbetuximab in combination with mFOLFOX6 chemotherapy. GLOW trial aims to evaluate the efficacy of zolbetuximab in combination with CAPOX compared to placebo with CAPOX.
LM-102, developed by LaNova, is a CLDN18.2 monoclonal antibody. In early 2021, LM-102 obtained FDA Investigational New Drug (IND) approval and successfully administered it to the first patient in the United States. The ongoing clinical trial (NCT04735796) is an open-label, dose-escalation Phase I/II study evaluating LM-102 injection’s safety, tolerability, pharmacokinetics, immunogenicity, and preliminary antitumor activity in patients with advanced solid tumors, including GC/GEJ, pancreatic cancer (PC), biliary tract cancer, esophageal adenocarcinoma, and ovarian mucous carcinoma.
In October 2021, LaNova initiated a Phase II clinical trial (NCT05008445) for LM-102 in China. This study aims to assess the safety, tolerability, pharmacokinetics, immunogenicity, and antitumor activity of LM-102 monotherapy or combination therapy in patients with CLDN18.2-positive advanced solid tumors. The trial is a multicenter collaboration led by Zhongshan Hospital, Fudan University. However, the current status of this clinical study is listed as terminated, and the specific reasons have not been disclosed.
ASKB589, developed by AOSAIKANG PHARM, is a humanized Claudin18.2 IgG1 monoclonal antibody. It primarily exerts its effects through antibody-dependent cell-mediated cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC), effectively targeting tumor cells. In July 2020, ASKB589 received clinical application approval from the National Medical Products Administration (NMPA) in China. The ongoing Phase Ib/IIa clinical trial (NCT05632939) evaluates ASKB589 in combination with CAPOX (capecitabine and oxaliplatin) and a PD-1 inhibitor as a first-line treatment for patients with CLDN18.2-positive, inoperable, locally advanced, recurrent, or metastatic gastric and gastroesophageal junction (G/GEJ) adenocarcinoma. The approval of this study marks a significant milestone in the exploration of ASKB589 as a critical therapeutic option for gastric cancer.
AB011, developed by CARSGEN THERAPEUTICS, is a recombinant humanized Claudin 18.2 monoclonal antibody used for treating CLDN18.2-positive solid tumors. It is the first domestically developed monoclonal antibody targeting this specific protein and also the first humanized monoclonal antibody worldwide to do so. Preclinical studies have demonstrated significant antitumor effects. Currently, AB011 is undergoing a Phase I clinical trial (NCT04400383) in China for the treatment of CLDN18.2-positive advanced solid tumors.
MIL93, developed by Mabworks, is a recombinant humanized anti-Claudin 18.2 IgG1 monoclonal antibody. It is being developed for the treatment of CLDN18.2-positive gastric cancer and pancreatic cancer. The company has modified the Fc segment of MIL93 to enhance its affinity for FcγRIIIa receptors on NK cells, resulting in an enhanced ADCC effect compared to similar candidate drugs. Currently, MIL93 is undergoing a Phase I clinical trial (NCT04671875) in China to evaluate its safety, tolerability, pharmacokinetics, and preliminary efficacy in patients with advanced or metastatic solid tumors. The study is currently recruiting participants.
M108, developed by FutureGen, is a recombinant humanized monoclonal antibody targeting Claudin 18.2. It is the first domestically developed monoclonal antibody specifically designed for CLDN18.2-positive gastrointestinal cancers, including gastric cancer. M108 is an ADCC-enhanced antibody, meaning it effectively engages immune cells to target tumor cells. M108 demonstrated high target specificity, strong antitumor activity, and a favorable safety profile in In Vitro and In Vivo Studies. The ongoing Phase I Clinical Trial (NCT04894825) aims to evaluate the safety, tolerability, pharmacokinetics, immunogenicity, and preliminary efficacy of M108 monotherapy and M108 combined with chemotherapy, which is currently recruiting participants.
QL1779, developed by QILU PHARMACEUTICAL, is a recombinant humanized Claudin 18.2 monoclonal antibody. In April 2023, QL1779 received implicit approval from the NMPA in China for the treatment of solid tumors.
TST001, also known as Osemitamab, is a second-generation humanized antibody targeting CLDN18.2 developed by TRANSCENTA. It exhibits improved CLDN18.2 binding affinity and enhanced ADCC. In preclinical tumor models with widespread CLDN18.2 expression, Osemitamab demonstrated antitumor activity. At the 2023 ASCO Annual Meeting and ESMO GI Congress, encouraging efficacy data were presented for Osemitamab in combination with CAPOX as a first-line treatment for GC/GEJ. In October 2023, the FDA approved the initiation of the TranStar 301 global Phase III pivotal trial (NCT06093425), evaluating Osemitamab in combination with nivolumab and chemotherapy for CLDN18.2-positive, inoperable, locally advanced, recurrent, or metastatic GC/GEJ. The study is currently in the recruitment phase.
NBL-015, developed by Shanghai Newsummit Biopharma, is a fully human anti-Claudin-18.2 monoclonal antibody. Engineered for optimized ADCC, CDC, and ADCP effects, NBL-015 exhibits low immunogenicity, good safety, strong affinity, and high antitumor activity compared to similar drugs. A Phase I clinical trial (NCT05153096) is currently evaluating the safety, tolerability, pharmacokinetics, and preliminary efficacy of NBL-015 injection in patients with advanced solid tumors. The study has not yet commenced patient recruitment.
3.2 Claudin18.2 Bispecific Antibody
Q-1802 is a bispecific antibody developed by QureBio Ltd. It targets both the tumor-specific antigen Claudin 18.2 and the immune checkpoint PD-L1. This innovative antibody has a dual mechanism of action: Q-1802 mediates effector cell killing of tumor cells through the Claudin 18.2 antibody. Simultaneously, Q-1802 blocks the PD-1 signaling pathway by binding to PD-L1, thereby activating both innate and adaptive immune responses. In December 2020, the NMPA accepted the clinical trial application for Q-1802. Notably, Q-1802 became the world’s first Claudin 18.2/PD-L1 bispecific antibody to receive FDA IND approval. QureBio Ltd. is currently conducting two clinical trials for Q-1802: NCT04856150 (Phase I) and NCT05964543 (Phase I/II).
ABL-111, also known as TJ-CD4B or TJ033721, is an innovative bispecific antibody developed by I-MAB Biopharma. It simultaneously targets Claudin 18.2 and 4-1BB. Even in cases of low Claudin 18.2 expression, ABL-111 can still bind to tumor cells and exhibit superior immune activity compared to other 4-1BB monoclonal antibodies. ABL-111 possesses a unique 4-1BB binding epitope, activating T cells only when bound to Claudin 18.2, thus avoiding excessive T cell activation due to widespread 4-1BB expression and reducing the risk of hepatotoxicity and systemic immune reactions. Currently, Tianjin Bio is conducting a Phase I clinical trial in the United States (ClinicalTrials.gov identifier: NCT04900818). This trial aims to evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of ABL-111 in patients with advanced or metastatic solid tumors.
AMG910 is a half-life extended (HLE) bispecific T-cell engager (BiTE) antibody that targets CD3-positive T cells and CLDN18.2-expressing tumor cells. It has been developed jointly by Amgen and BeiGene. This innovative antibody can be used for the research of adenocarcinoma. Currently, AMG910 is undergoing a global multicenter Phase I clinical trial for the treatment of GC/GEJ (NCT04260191). The trial aims to evaluate the efficacy and safety of AMG910 in patients with CLDN18.2-positive gastric cancer.
3.3 Claudin18.2 ADC
LM-302 is a first-in-class investigational drug developed by LaNova. It is an ADC based on the Claudin 18.2 antibody. LM-302 specifically targets Claudin 18.2-positive tumor cells and enters these cells through endocytosis. Once inside, it releases small-molecule toxins, exerting its anti-tumor effects. Preclinical research data for LM-302 demonstrate favorable safety profiles and in vivo and in vitro activity. Notably, it shows promising efficacy even in tumor models with low Claudin 18.2 expression. In June 2021, LM-302 received FDA IND clearance in the United States and was granted orphan drug designation by the FDA for PC, GC/GEJ. On October 14, 2021, LM-302 also obtained China NMPA IND approval. Currently, there are three ongoing clinical trials, with the highest clinical stage reported on ClinicalTrials.gov being Phase II. However, it’s worth noting that LaNova’s official website indicates a Phase III stage for this innovative antibody-drug conjugate.
SYSA1801 is a fully human anti-Claudin-18.2 monoclonal antibody-MMAE drug conjugate developed by CSPC. It specifically targets Claudin 18.2. In preclinical studies, SYSA1801 demonstrated significant in vitro and in vivo anti-tumor activity in multiple cell lines and xenografts expressing CLDN18.2. The antibody effectively targets tumor cells through anti-Claudin-18.2 binding and triggers endocytosis, delivering the MMAE toxin specifically into tumor cells for the treatment of gastric cancer and pancreatic cancer.
The ongoing clinical trial (NCT05009966) is an open-label, multi-center, Phase I study evaluating the safety, tolerability, pharmacokinetic characteristics, immunogenicity, and preliminary efficacy of SYSA1801 in patients with advanced malignant solid tumors expressing CLDN18.2 (defined as CLDN18.2 IHC≥1+). Notably, preliminary results presented at the 2023 ASCO Annual Meeting by Dr. Yakun Wang from the Peking University Cancer Hospital & Institute showed promising efficacy and safety in the first-in-human study of SYSA1801 for patients with recurrent or refractory advanced solid tumors expressing CLDN18.2, particularly in gastric cancer patients.
CMG901, also known as AZD0901, is an ADC developed by Keymed Biosciences. It specifically targets Claudin 18.2 and consists of a Claudin 18.2-specific antibody, a cleavable linker, and MMAE. CMG901 is intended for the treatment of solid tumors, including gastric cancer and gastroesophageal junction (GEJ) adenocarcinoma. Currently, it is in Phase I clinical trials. At the November 2023 ASCO meeting, Keymed presented the latest data from the Phase I clinical trial (NCT04805307) evaluating CMG901 in patients with advanced gastric or GEJ adenocarcinoma. The study aims to assess the safety, tolerability, pharmacokinetic characteristics, immunogenicity, and preliminary efficacy of CMG901 in these patients. Notably, CMG901 demonstrated favorable safety profiles and tolerability, with most adverse events manageable and allowing continued treatment. In patients with Claudin18.2-positive advanced gastric or GEJ adenocarcinoma, CMG901 showed promising efficacy.
ATG-022, also known as AZD0901, is a humanized IgG1 ADC developed by Antengene. It specifically targets the Claudin 18.2 antigen and is intended for the treatment of solid tumors. Due to its unique mechanism of action and high specificity for tumor cells, the company believes that ATG-022 has the potential for combination therapy with other pipeline assets and can serve as part of a tumor-targeting bispecific antibody group. In March 2023, the clinical trial for ATG-022 received approval in China. The Phase I CLINCH study aims to evaluate the safety, pharmacokinetic characteristics, and preliminary efficacy of ATG-022 as a monotherapy in patients with advanced or metastatic solid tumors expressing CLDN18.2. Preliminary results from this study demonstrated good safety and tolerability, with most adverse events manageable and allowing continued treatment.
3.4 Claudin18.2 CAR-T
CT041, developed by CARSGEN, is a humanized anti-Claudin18.2 autologous CAR-T cell therapy. In May 2020, CT041’s IND application received approval from the U.S. FDA, making it the first-in-class globally for CAR-T cell candidate targeting the Claudin18.2 antigen. In August 2020, CT041 was granted its first clinical approval in China. Currently, the CT041 autologous CAR-T cell infusion is being evaluated in a Phase Ib/II clinical trial for patients with advanced GC/GEJ and PC.
Data presented at the September 2021 ESMO meeting demonstrated favorable safety and promising anti-tumor activity of CT041 in patients with refractory CLDN18.2-positive gastrointestinal tumors. Additionally, on January 19, 2024, CARSGEN showcased a clinical study of CT041 (satri-cel), a Claudin18.2-targeted autologous CAR-T cell therapy, at ASCO GI. The study primarily reported results from the ELIMYN18.2 Phase 1b dose-escalation cohort (Cohort A) conducted in the United States for treating GC/GEJ or PC. These results indicated promising preliminary efficacy in previously treated Claudin18.2-positive advanced GC/GEJ and PC patients.
LB1908 is an investigational, autologous chimeric antigen receptor T-cell (CAR-T) therapy selectively targeting Claudin 18.2 through a high-affinity VHH antibody developed by Legend Biotech. This VHH antibody specifically binds to Claudin 18.2 with high affinity and exhibits cytotoxicity only against cells expressing CLDN18.2, while sparing human primary cells or cells expressing Claudin 18.1. Currently, LB1908 has entered a Phase I clinical trial (NCT05539430) for the treatment of gastric cancer or PC. This innovative CAR-T cell therapy holds promise as a targeted approach for patients with CLDN18.2-positive tumors.
4. DIMA Biotech: Advancing Claudin18.2 Biotherapy Development
DIMA Biotech is a specialized biotechnology company focusing on preclinical research products and services for actionable drug targets. DIMA now offers a comprehensive range of products and services related to the Claudin18.2 target. The product includes active recombinant proteins, Biosimilar antibodies, and FC-validated monoclonal antibodies. Their services encompass various protein antibody customization services, antibody humanization, and affinity maturation services. To expedite the development of Claudin18.2 biotherapy, DIMA Biotech has established a Claudin18.2 target single B-cell seed library. This resource allows for the rapid acquisition of lead antibody molecules in as little as 28 days. Additionally, DIMA Biotech has undertaken CAR-T or ADC molecule construction and functional validation for some existing Claudin18.2 lead antibody molecules. For specific data inquiries and further information, feel free to contact us.
- Featured Product
Fluorescent Human CLDN18.2 Full Length Protein-VLP (EGFP) (FLP100017)
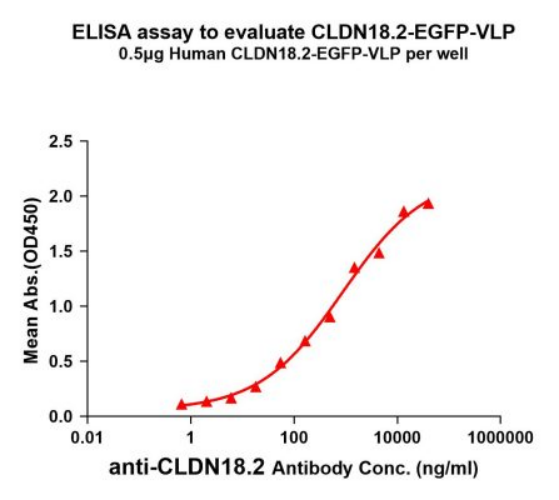
Figure 2. ELISA plates were pre-coated with 0.5μg/per well-purified human CLDN18.2-EGFP-VLP. Serial diluted Anti-CLDN18.2 monoclonal antibody (BME100075) solutions were added, washed, and incubated with a secondary antibody before ELISA reading. From the above data, the EC50 for anti-CLDN18.2 monoclonal antibody binding with CLDN18.2-EGFP-VLP is 877.5ng/ml.
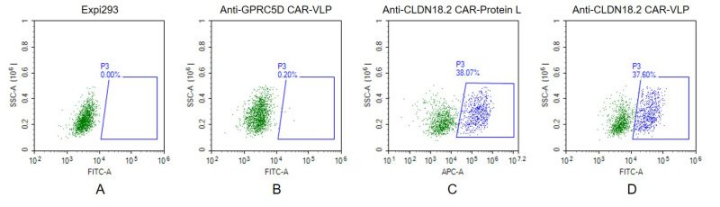
Figure 3. FACS analysis of CLDN18.2 VLP.
A. Negative Control 1: HEK293 cells were stained with Fluorescent Human CLDN18.2 Full Length Protein-VLP (EGFP).
B. Negative Control 2: Anti-GPRC5D-CAR-HEK293 cells (an irrelevant CAR) were stained with Fluorescent Human CLDN18.2 Full Length Protein-VLP (EGFP).
C. Positive Control: Anti-CLDN18.2-CAR-HEK293 cells were stained with biotin-labeled Protein L, followed by streptavidin-APC antibody.
D. Anti-CLDN18.2-CAR-HEK293 cells were stained with Fluorescent Human CLDN18.2 Full Length Protein-VLP (EGFP).
- The full products of Claudin18.2
| Product Types | SKU | Product Name |
| ECD Proteins | PME-C100050 | Cynomolgus CLDN18.2 Protein, hFc Tag |
| Full Length Transmembrane Proteins | FLP100006 | Human CLDN18.2 full length protein-VLP |
| FLP100014 | Human CLDN18.2 full length protein-synthetic nanodisc | |
| FLP100017 | Fluorescent Human CLDN18.2 Full Length Protein-VLP (EGFP) | |
| FLP200014 | Human CLDN18.2 full length protein-MNP | |
| FLP100014B | Biotinylated Human CLDN18.2 full length protein-synthetic nanodisc | |
| FLP120014 | Human CLDN18.2-Strep full length protein-synthetic nanodisc | |
| Monoclonal Antibodies | DME100179 | Anti-CLDN18.2 antibody(DM179); Rabbit mAb |
| DME100179B | Biotinylated Anti-CLDN18.2 antibody(DM179); Rabbit mAb | |
| DME100179P | PE-conjugated Anti-CLDN18.2 antibody(DM179); Rabbit mAb | |
| Biosimilar reference antibodies | BME100075 | Anti-CLDN18.2 (zolbetuximab biosimilar) mAb |
| BME100075B | Biotinylated Anti-CLDN18.2 (zolbetuximab biosimilar) mAb | |
| BME100075P | PE-conjugated Anti-CLDN18.2 (zolbetuximab biosimilar) mAb |
- The progress of DIMA’s Claudin18.2 Lead Molecular

