At the end of 2020, Genmab and ADC Therapeutics announced the termination of clinical development for Enapotamab vedotin and ADCT-601, respectively, due to their failure to meet expected outcomes in early trials. Both drugs were antibody-drug conjugates (ADCs) targeting AXL. Enapotamab vedotin was coupled with the microtubule inhibitor MMAE, while ADCT-601 was coupled with the PBD dimer cytotoxin. In January 2023, BioAtla released the latest clinical data for BA-3011 (CAB-AXL-ADC), showing no significant positive effects. Following this data release, BioAtla’s stock price dropped by 46%. BA-3011 is an ADC designed to target AXL based on CAB technology.
Currently, there are a total of 29 clinical drugs targeting AXL, including 3 ADCs, 1 CAR-T, 1 antibody, and 24 small-molecule drugs (including 2 already on the market). The challenges faced by AXL-ADC drugs have created difficulties for AXL-targeted immunotherapy. What is the mechanism of action of the AXL target, and how is the landscape of AXL-targeted immunotherapy shaping up?
1. The Structure and Distribution of AXL
AXL, also known as Ark, Ufo, and Tyro-7, is a receptor tyrosine kinase that belongs to the TAM family, which also includes TYRO3 and MERTK. It was first discovered in 1988 by Liu et al. when they screened genes associated with chronic myeloid leukemia (CML) [1]. The human AXL gene is located on chromosome 19q13.2 and has 20 exons encoding a protein with 894 amino acids (MW 104 kDa). AXL protein contains extracellular, transmembrane, and intracellular regions [2].
The extracellular region is encoded by exons 1-10, including the signal peptide, two immunoglobulin (Ig) domains and two fibronectin type III (FNIII) domains; extracellular proteolytic cleavage sites and a transmembrane domain are encoded by exon 11; exons 12-20 encode the intracellular domain with tyrosine kinase activity. The intracellular domain is crucial for kinase activity after autophosphorylation and activation, and can participate in the transmission of various signals in normal cells and tumor cells [3].
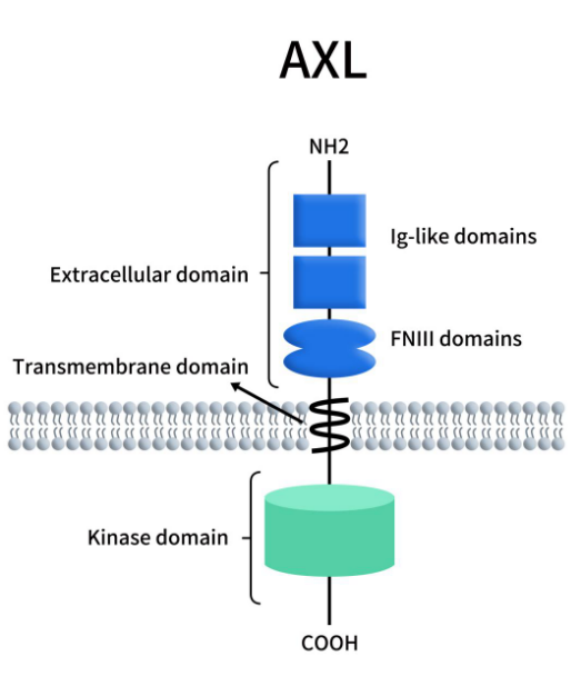
Figure 1. The structure of AXL [4]
Under normal physiological conditions, AXL expression is mostly limited to smooth muscle, lungs, kidneys, testes, and blood cells [5]. However, in various cancers such as non-small cell lung cancer (NSCLC), breast cancer, and ovarian cancer, AXL is highly expressed, closely associated with tumorigenesis, progression, and drug resistance. Therefore, AXL is considered as a potential target for cancer therapy, and several strategies have been developed to inhibit its activity or expression
2. AXL Signaling Pathway
As mentioned earlier, AXL, like other TAM family receptors, responds to various biological processes, including inflammatory responses, by typically integrating extracellular signals through the classic binding of ligands. This integration induces effective intracellular signal transduction. AXL activation occurs through two main methods: one is the predominant classic ligand-dependent activation, and the other is non-Gas6-dependent activation. The classic ligand-dependent activation of AXL refers to the AXL/Gas6 signaling pathway. The non-Gas6-dependent activation of the AXL receptor involves interactions with two other TAM receptors (Mer and Tyro-3) and several non-TAM family member proteins (such as EGFR, HER2, etc.). Here, we will focus on the primary AXL/Gas6 signaling pathway.
As illustrated below, when the Ig domain of AXL binds with GAS6, a complex forms. This complex undergoes dimerization with another GAS6-AXL complex, creating a 2:2 homologous dimerized complex. After the AXL receptor dimerizes, tyrosine residues in the intracellular domain of the AXL receptor undergo transphosphorylation in a counter-clockwise manner. The AXL intracellular kinase domain has a total of 6 phosphorylation sites, where 3 C-terminal sites (Y698, Y702, and Y703) are conserved in TAM. Additionally, the other 3 N-terminal sites (Y779, Y821, and Y866) undergo autophosphorylation and participate in docking and activation. The phosphorylation of tyrosine residues in the AXL intracellular domain is necessary to recruit intracellular adapter molecules and effector proteins, ultimately activating downstream signal pathways. AXL activation initiates the signal transduction of various downstream pathways, such as PI3K/AKT, MAPK/ERK, and PKC. The activation of these signal transductions plays diverse roles in cellular activities, including cell proliferation and survival, migration and invasion, epithelial-mesenchymal transition (EMT), angiogenesis, therapy resistance, immune suppression, and the activation of stem cell maintenance functions [6].
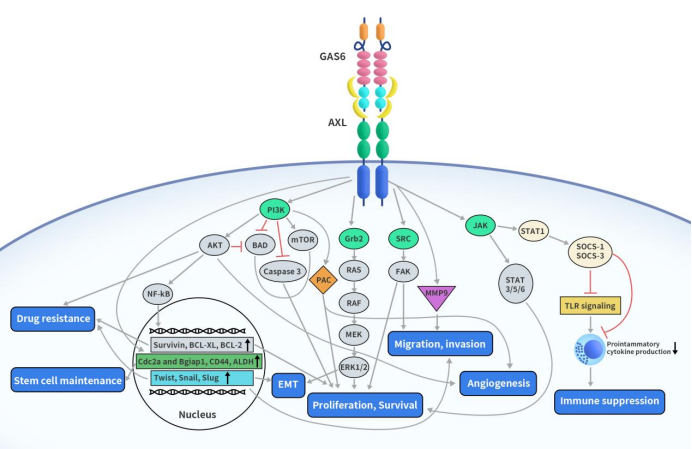
Figure 2 AXL signaling pathway [3]
3. Progress in AXL Targeted Therapy
Clinical trial data show that current AXL-targeted drugs primarily consist of small molecules, with only 5 based on immunotherapy: 3 ADCs, 1 CAR-T, and 1 antibody.
3.1 AXL-ADC
Enapotamab vedotin, also known as AXL-107-MMAE, is an antibody-drug conjugate (ADC) targeting AXL with a DAR value of 4. Developed by Genmab, it uses a cleavable valine-alanine linker to connect AXL-specific IgG1 with monomethyl auristatin E (MMAE), a connection technology licensed from Seagen Inc.. The clinical focus of Enapotamab vedotin is on solid tumors, including ovarian cancer, non-small cell lung cancer (NSCLC), endometrial cancer, cervical cancer, and thyroid cancer. It is currently in the advanced stage of clinical phase II. Preclinical studies indicate significant anti-tumor activity of Enapotamab vedotin in NSCLC models [7]. Unfortunately, the effectiveness of Enapotamab vedotin did not align with the dose and biomarker criteria set by Genmab for continued development, leading to its clinical development termination announced by Genmab on November 25, 2020.
Mipasetamab uzoptirine, also known as ADCT-601, is an AXL-targeting antibody-drug conjugate consisting of a humanized anti-AXL IgG1 antibody, a valine-alanine cleavable linker, and the potent pyrrolobenzodiazepine (PBD) dimer cytotoxin SG3199. Developed by ADC Therapeutics, ADCT-601 has a DAR value of 2. When ADCT-601 binds to cells expressing AXL protein, it undergoes internalization and releases PBD, which further enters the cell nucleus and binds with DNA.
Preclinical studies of ADCT-601 show significant and sustained anti-tumor activity in various human cancer xenograft mouse models [8]. At the 2019 ESMO conference, ADC Therapeutics disclosed preliminary Phase I clinical results for ADCT-601: in terms of safety, 13 patients received treatment at doses ranging from 50 to 150 µg/kg every 3 weeks, including 3 at 50 µg/kg, 6 at 100 µg/kg, and 4 at 150 µg/kg. One patient treated at 100 µg/kg experienced dose-limiting toxicity with grade 3 hematuria. All subjects encountered treatment-related adverse events (TEAE), with 7 experiencing 3-grade or more than 3-grade TEAE. ADC Therapeutics announced the termination of the ADCT-601 clinical trial at the end of 2020.
However, based on clinical trial data, ADC Therapeutics initiated a new clinical trial, CT05389462 in 2022 for ADCT-601. Unlike the previously terminated CT03700294, the primary objective of CT05389462 is to determine the Phase II recommended dose (RP2D) and/or maximum tolerated dose (MTD) of ADCT-601, validating its safety and tolerability as a single therapy and in combination with gemcitabine. This clinical trial is currently in the recruiting phase, with no available data as of now.
Mecbotamab vedotin, also known as BA-3011, is an AXL receptor-targeted antibody-drug conjugate developed by the biotechnology company BioAtla in San Diego, USA, using the conditionally active biologics (CAB) platform. The antibody component, after modification through CAB, selectively targets the AXL receptor in tumor tissues, connecting to the microtubule inhibitor drug MMAE, with a DAR value of 4. The mid-term analysis results of the Phase II clinical trial for NSCLC, announced in January of this year, showed that out of 10 patients treated with BA-3011 as a monotherapy, 4 exhibited a response, resulting in an overall response rate (ORR) of 40%. In the case of combined treatment with BA3011 and Opdivo in 8 patients, only 1 achieved partial response (PR), leading to an ORR of 13%. Following the release of this data, BioAtla’s stock price experienced a significant drop of 46%.
3.2 AXL-CAR-T Therapy
CCT301-38 AXL, also known as CCT301-38, is a CAR-T product targeting AXL developed by exumabio (formerly F1 Oncology) company. CCT301-38 AXL is processed using the third-generation suspension lentivirus system developed by F1 Oncology and a specialized T-cell encapsulation cell processing system. It is currently in the advanced clinical stage of Phase II, targeting indications such as renal cell carcinoma and seasonal allergic rhinitis.
3.3 AXL-Antibody
Tilvestamab, also known as BGB-149, is a fully humanized AXL inhibitory antibody developed by Bergenbio. It primarily binds to the IgG1 structure of AXL’s extracellular domain, consequently blocking the interaction between GAS6 and AXL. Tilvestamab has demonstrated anti-tumor activity in models of AML, NSCLC, pancreatic cancer, and renal cancer. Currently, it is undergoing a Phase I clinical trial targeting ovarian cancer.
4. Advancing Drug Development with DIMA’s AXL Solutions
DIMA Biotech is a biotechnology company specializing in providing pre-clinical research products and services for potential drug targets. Through innovative platforms for membrane protein production, single B-cell lead antibody discovery, antibody engineering, and functional verification platforms, DIMA offers a comprehensive range of AXL products and services. The product portfolio includes active proteins for various species, flow-validated monoclonal antibodies, and benchmark reference antibodies. DIMA also provides tailored antibody services, including antibody humanization, affinity maturation, and removal of post-translational modification (PTM) risk sites to enhance antibody optimization. To expedite AXL-targeted drug development, DIMA has established a B-cell seed library for AXL, enabling the completion of lead antibody molecule screening in as little as 20 days.
- AXL Recombinant Proteins (from different species):
More AXL Recombinant Proteins (with different tags)
Human AXL Protein, mFc Tag (PME100533)
Human AXL Protein, hFc Tag ( PME100532)
- Rabbit Monoclonal Antibody
Anti-AXL antibody(DM158); Rabbit mAb (DME100158)
- Benchmark Antibody for AXL with FC Data
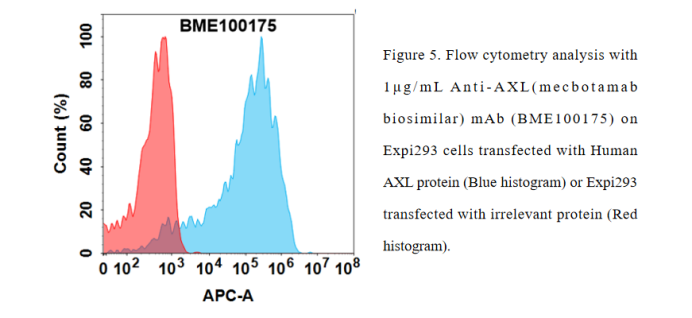
Anti-AXL(mecbotamab biosimilar) mAb ( BME100175)
More Benchmark Antibody for AXL Protein:
Anti-AXL (enapotamab biosimilar) mAb (BME100033)
Reference:
[1] Liu E, Hjelle B, Bishop JM. Transforming genes in chronic myelogenous leukemia. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1952-6.
[2] O’Bryan JP, Frye RA, Cogswell PC, Neubauer A, Kitch B, Prokop C, Espinosa R 3rd, Le Beau MM, Earp HS, Liu ET. axl, a transforming gene isolated from primary human myeloid leukemia cells, encodes a novel receptor tyrosine kinase. Mol Cell Biol. 1991 Oct;11(10):5016-31.
[3] Tang Y, Zang H, Wen Q, Fan S. AXL in cancer: a modulator of drug resistance and therapeutic target. J Exp Clin Cancer Res. 2023 Jun 16;42(1):148.
[4] Zhu C, Wei Y, Wei X. AXL receptor tyrosine kinase as a promising anti-cancer approach: functions, molecular mechanisms and clinical applications. Mol Cancer. 2019 Nov 4;18(1):153.
[5] Uhlén M, Fagerberg L, Hallström BM, et al. Tissue-based map of the human proteome. Science. 2015 Jan 23;347(6220):1260419.
[6] olavito SA. AXL as a Target in Breast Cancer Therapy. J Oncol. 2020 Feb 14;2020:5291952.
[7] Koopman LA, Terp MG, Zom GG, et al. Enapotamab vedotin, an AXL-specific antibody-drug conjugate, shows preclinical antitumor activity in non-small cell lung cancer. JCI Insight. 2019 Nov 1;4(21):e128199.
[8] Zammarchi F, Havenith KE, Chivers S, et al. Preclinical Development of ADCT-601, a Novel Pyrrolobenzodiazepine Dimer-based Antibody-drug Conjugate Targeting AXL-expressing Cancers. Mol Cancer Ther. 2022 Apr 1;21(4):582-593.
