Acute myeloid leukemia (AML) is a complex and highly heterogeneous hematologic malignancy closely associated with various cytogenetic and chromosomal abnormalities. Among them, mutations in the FMS-like tyrosine kinase 3 (FLT3) gene are one of the most common genetic alterations. Studies have shown that approximately 25% of AML patients carry FLT3 internal tandem duplication mutations (FLT3-ITD), while about 10% have FLT3 tyrosine kinase domain mutations (FLT3-TKD). These mutations typically lead to persistent activation of the FLT3 signaling pathway, promoting abnormal proliferation of leukemic cells and correlating with poor prognosis.
Although AML patients with FLT3 mutations may achieve temporary remission following conventional chemotherapy or hematopoietic stem cell transplantation (HSCT), they face a higher risk of relapse and more significant treatment challenges. Therefore, FLT3 has emerged as a critical target for AML research and therapy, and targeted treatment strategies against this receptor are continuously evolving and being optimized. In the following, we will delve into the FLT3 receptor and its role in AML.
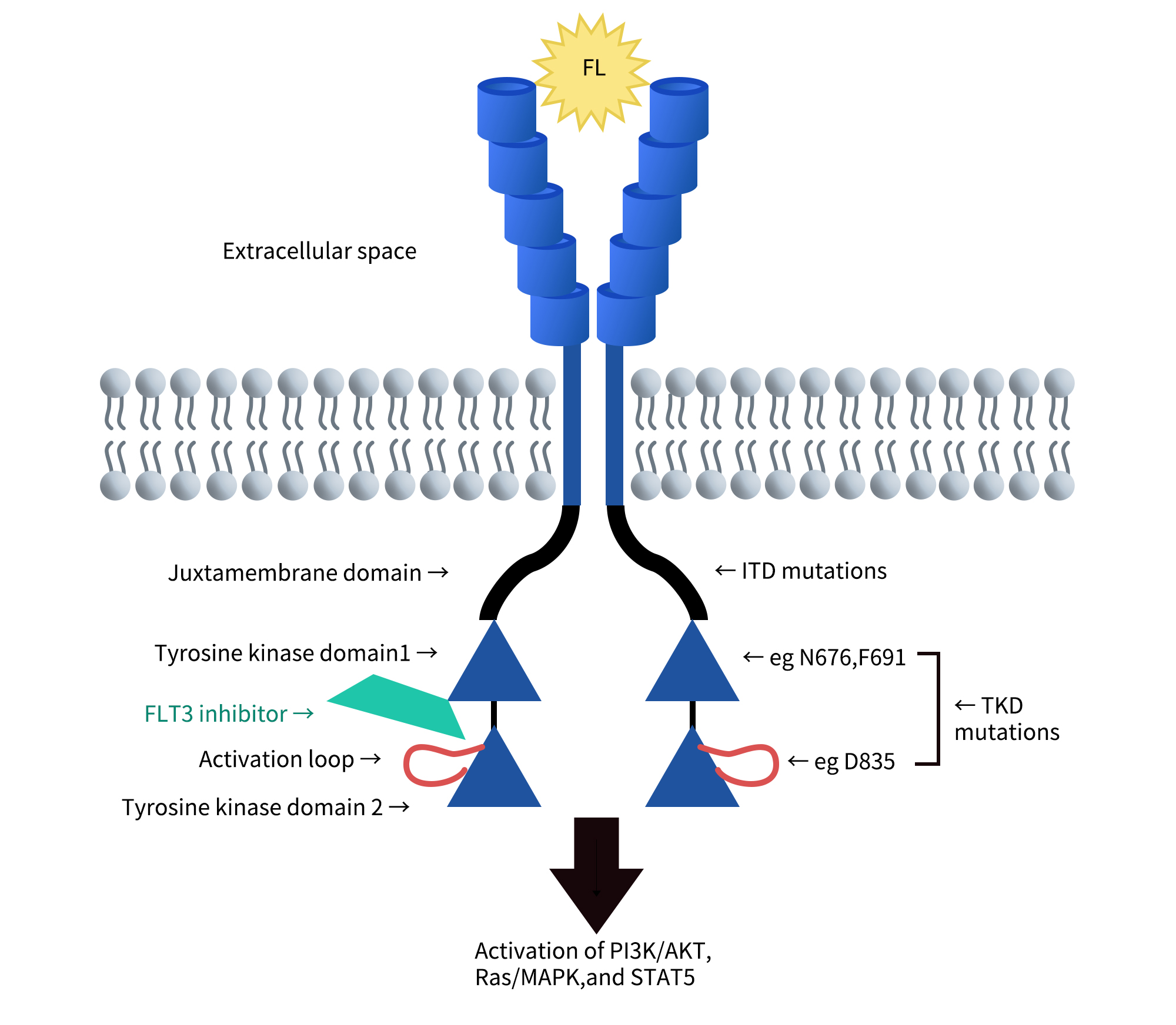
1. FLT3 Structure and Expression
FMS-like tyrosine kinase 3 (FLT3) belongs to the class III receptor tyrosine kinase (RTK) family, along with KIT, FMS, and platelet-derived growth factor receptors (PDGFR).
Its structure includes:
- Extracellular domain: Composed of five immunoglobulin-like regions at the N-terminus, responsible for ligand binding.
- Transmembrane domain: Anchors the receptor to the cell membrane, ensuring stable signal transmission.
- Juxtamembrane domain (JMD): Plays a regulatory role during receptor activation.
- Tyrosine kinase domain: Composed of two kinase domains separated by a kinase insert region; it is a critical component for downstream signal transduction.
FLT3 is primarily expressed in hematopoietic stem cells (CD34⁺cells) and early progenitor cells in the bone marrow (BM), where it plays a role in cell proliferation and differentiation. It is also expressed in dendritic cell progenitors. Additionally, FLT3 is found in tissues such as the placenta, brain, and testes, although its function in these tissues remains unclear.
The FLT3 gene is located on chromosome 13q12 and comprises 24 exons. The protein exists in two forms:
- Membrane-bound form (158–160 kDa): Modified with N-linked glycosylation.
- Cytoplasmic form (130–143 kDa): Unmodified by glycosylation.
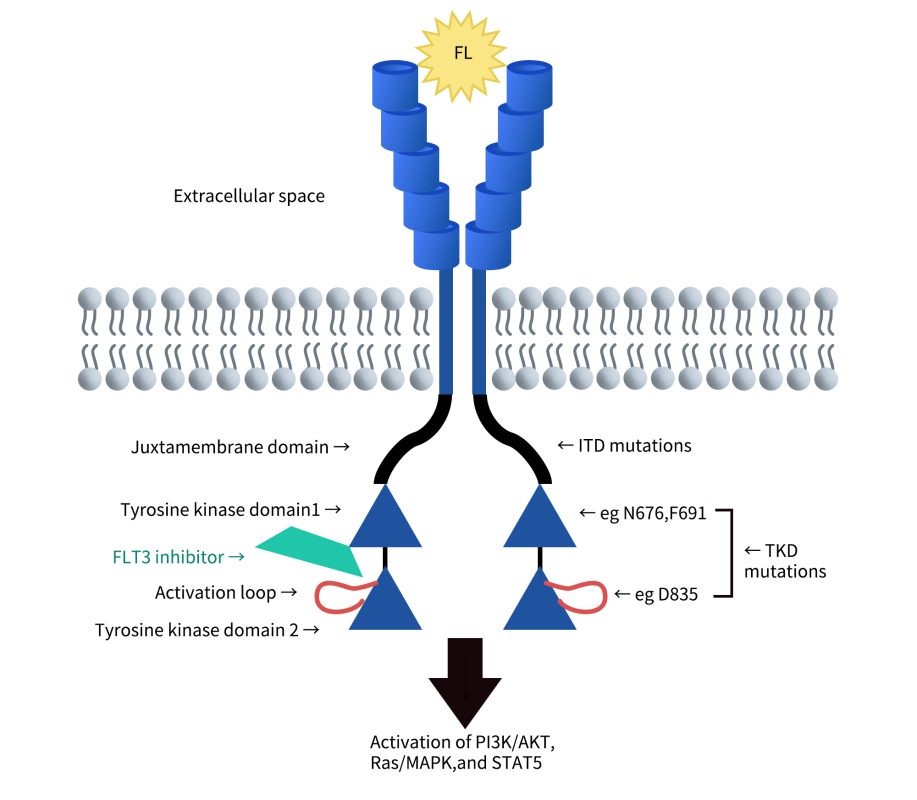
Cite from The biology and targeting of FLT3 in pediatric leukemia.
The natural ligand of FLT3, FL (FLT3 ligand), is primarily expressed by bone marrow stromal cells and exists in a membrane-bound form. Upon binding to FLT3, FL induces receptor dimerization and autophosphorylation, subsequently activating intracellular signaling pathways that regulate the proliferation and differentiation of immature hematopoietic cells. Although FL alone has relatively weak proliferative activity, it can act synergistically with other cytokines, such as stem cell factor (SCF, the ligand for C-KIT), to enhance the expansion of progenitor cells. In addition, FL can promote the adhesion of hematopoietic progenitor cells to stromal cells via VLA-4 and VLA-5, playing a vital role in the hematopoietic process. The exact function of FLT3 in the central nervous system remains unclear, and further research is required to explore its physiological roles and potential impact in disease.
2. FLT3 Signal Transduction
FMS-like tyrosine kinase 3 (FLT3) is a receptor tyrosine kinase that is almost exclusively expressed within the hematopoietic compartment. Its ligand, FL, induces dimerization and activation of FLT3’s intrinsic tyrosine kinase activity. Activation of FLT3 leads to autophosphorylation and initiation of multiple downstream signaling cascades. Signal transduction occurs through the recruitment of signaling molecules to activated FLT3 via specific phosphorylated tyrosine residues located in its intracellular region.
FLT3 activation mediates cell survival, proliferation, and differentiation of hematopoietic progenitor cells. It exerts its biological functions in cooperation with other cytokines. Dysregulated FLT3 activity is associated with several diseases, most notably acute myeloid leukemia (AML), in which approximately one-third of patients harbor activating FLT3 mutations that drive disease progression and are linked to poor prognosis. Excessive FLT3 activity is also implicated in autoimmune diseases such as rheumatoid arthritis. The observation that gain-of-function mutations in FLT3 contribute to leukemogenesis has spurred the development of FLT3-targeted inhibitors. Many of these are currently in clinical trials, and some have already been approved for clinical use.
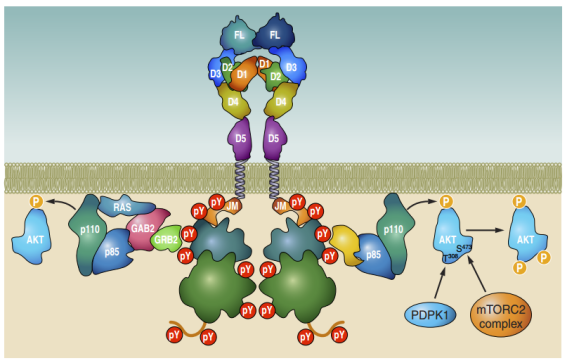
Cite from FMS-like Tyrosine Kinase 3/FLT3: From Basic Science to Clinical Implications.
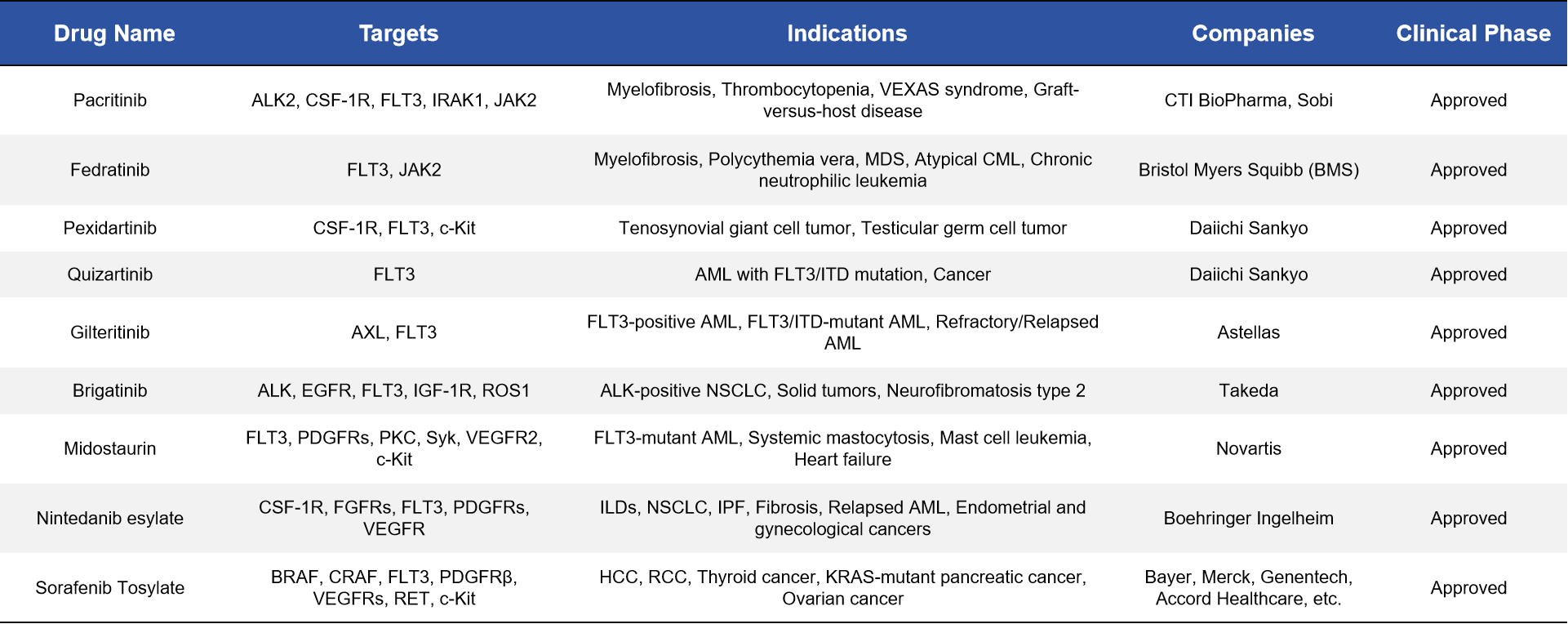
3. FLT3-Targeted Therapeutics in Clinical Use
In recent years, drug development targeting FLT3 has primarily focused on small molecule inhibitors. To date, nine such drugs have been approved for clinical use, all of which are small molecule inhibitors. In addition, several other candidates have advanced to phase III clinical trials. Beyond small molecule drugs, other therapeutic strategies targeting FLT3—including cell-based therapies (such as CAR-T and TCR-T), bispecific antibodies, and protein degradation technologies (such as PROTACs)—are under active investigation and have shown promising potential. Overall, FLT3 remains one of the most promising targets for AML treatment, with a rich drug development pipeline and diverse technological approaches.
3.1 Approved FLT3-Targeted Drugs
As of now, nine drugs with FLT3 inhibitory activity have been approved globally for the treatment of acute myeloid leukemia (AML) or other cancers. These drugs include:
- Selective FLT3 inhibitors, such as Gilteritinib, Quizartinib, and Crenolanib, which offer high specificity toward the FLT3 receptor.
- Multi-target tyrosine kinase inhibitors (TKIs), such as Midostaurin, Sorafenib, Sunitinib, Lestaurtinib, Tandutinib, and Fedratinib, which act on FLT3 as well as other kinases like VEGFR, PDGFR, and KIT.
These agents are primarily ATP-competitive small molecule inhibitors that block FLT3 autophosphorylation, thereby inhibiting downstream signaling pathways such as STAT5, MAPK, and PI3K/AKT. This mechanism suppresses the proliferation and survival of leukemic cells. The development of these drugs has not only expanded treatment options for patients with FLT3-mutant AML but also advanced the field of precision targeted therapy.
3.2 FLT3-Targeted Drugs in Clinical Development
Currently, FLT3-targeted drug development encompasses three major approaches: small molecule inhibitors, CAR-T cell therapies, and emerging PROTAC degraders. Among these, small molecule FLT3 inhibitors are the most extensively studied, with several candidates such as Crenolanib and Ruserontinib already in mid-to-late clinical stages, reinforcing their central role in the treatment of FLT3-mutant AML.
In the field of CAR-T therapy, although development is still in early clinical stages, innovative constructs like SENTI-202 are actively working to overcome safety challenges posed by the broad expression of the FLT3 target. In the realm of targeted protein degradation, LNK-01002 stands out as the first FLT3-targeted PROTAC drug to enter clinical trials, reflecting a growing trend of shifting from inhibition to degradation in drug development.
3.2.1 Small Molecule Inhibitors
Given FLT3’s status as a frequently mutated target in acute myeloid leukemia (AML), it has become a primary focus for small molecule drug development. According to incomplete statistics, there are currently more than 30 FLT3-targeted inhibitors in various clinical stages, highlighting the intensity and diversity of ongoing efforts in this field.
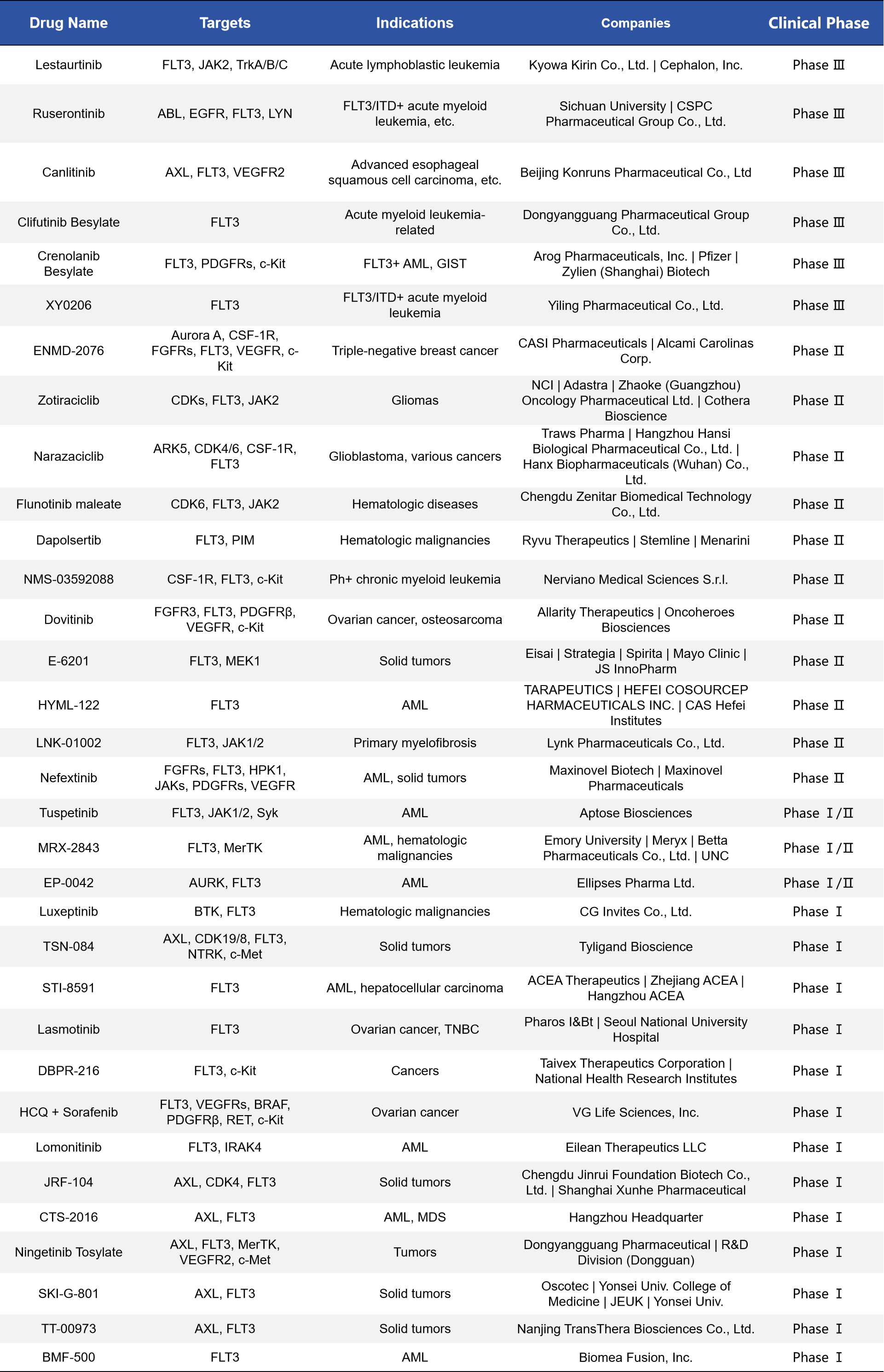
- Crenolanib: A second-generation FLT3 inhibitor with strong inhibitory activity against both FLT3-ITD and FLT3-TKD mutations. It also targets other receptor tyrosine kinases such as PDGFR and c-KIT. Currently in phase III clinical trials for AML, Crenolanib is being developed by Arog Pharmaceuticals. It has demonstrated superior efficacy and resistance profiles compared to first-generation inhibitors like Midostaurin.
- Ruserontinib: Developed primarily by Chinese companies, this is a multi-target kinase inhibitor that, in addition to FLT3, also inhibits ABL, EGFR, and LYN. It has reached phase III clinical trials, with its main indication being FLT3/ITD-mutant AML. Ruserontinib has shown strong clinical activity in this setting.
- ENMD-2076: A multi-target inhibitor with activity against Aurora A, FLT3, VEGFR, and others. It has shown potential in the treatment of triple-negative breast cancer (TNBC), indicating that FLT3 inhibitors are being explored not only for hematologic malignancies but also for solid tumors.
The continued development of these drugs is expanding treatment options for patients with FLT3-positive AML and other related diseases. At the same time, it highlights key research challenges, including metabolic stability, target selectivity, and mechanisms of resistance.
3.2.2 CAR-T Cell Therapies
Although CAR-T cell therapies targeting FLT3 are still in the early stages of development, they have shown promising potential in the treatment of relapsed/refractory AML. Currently, there are four investigational CAR-T therapies under development that specifically target FLT3.
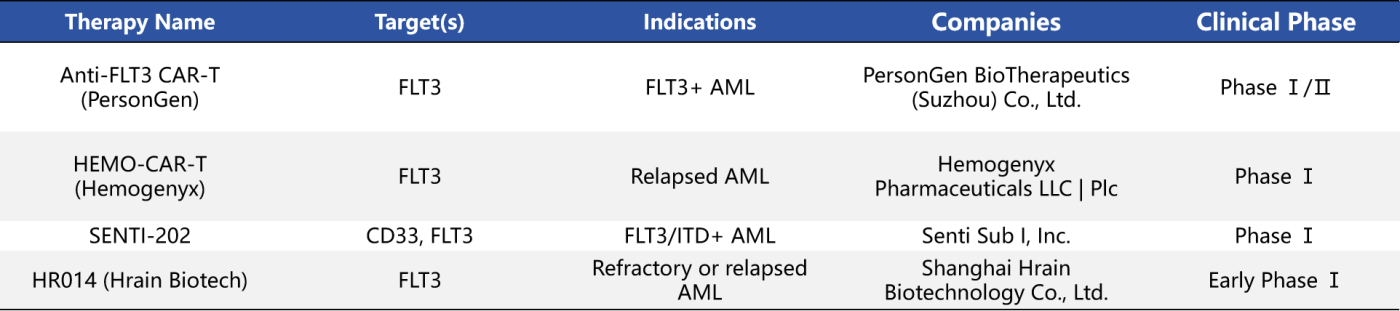
- Anti-FLT3 CAR-T: Designed for FLT3-positive AML patients, this therapy is currently in Phase I/II clinical trials. The product may incorporate strategies that combine the targeted elimination of leukemic stem cells with enhanced antigen specificity.
- SENTI-202: Developed by Senti Biosciences, this CAR-T therapy utilizes logic-gated control technology to simultaneously target CD33 and FLT3, aiming to improve treatment selectivity while minimizing toxicity to normal hematopoietic stem cells. This innovative design holds promise for addressing key challenges of traditional CAR-T therapies, such as off-target toxicity and cytokine release syndrome.
Although CAR-T products for AML face significant safety challenges due to the widespread expression of hematopoietic antigens, these early studies lay a crucial foundation for the future development of FLT3-targeted immune cell therapies.
3.2.3 PROTAC (Proteolysis-Targeting Chimera)
Currently, there is only one FLT3-targeted PROTAC drug in clinical development:

- LNK-01002: A dual-targeting PROTAC molecule designed to degrade both FLT3 and JAK1/2, LNK-01002 is being developed for the treatment of myeloid disorders, including primary myelofibrosis. Currently in Phase II clinical trials, this drug aims to overcome resistance to traditional inhibitors by inducing ubiquitin-mediated degradation of FLT3 protein.
Compared with traditional kinase inhibitors, PROTAC molecules achieve target elimination rather than mere functional inhibition, offering the potential for long-lasting efficacy and resistance mitigation in patients with FLT3 mutations.
4. DIMA Biotechnology’s FLT3-Related Products
DIMA Biotechnology LLC is a biotechnology company focused on the preclinical development of druggable targets and offers a variety of FLT3-related products currently in stock. These include recombinant proteins, recombinant monoclonal antibodies, and biotin/PE-labeled antibodies. In addition, DIMA provides comprehensive services such as custom protein/antibody production, antibody humanization, affinity maturation, and stable cell line development.
DIMA has established a prevalidated B cell library targeting human FLT3, enabling rapid screening of lead antibody candidates within just 20 days. For more information, please feel free to contact us.
- Proteins & Antibodies
| Type | SKU | Product Name |
| ECD Protein | PME100033 | Human FLT3 Ligand Protein, mFc-His Tag |
| PME100007 | Human FLT3 Protein, hFc-His Tag | |
| Monoclonal Antibodies | DMC100421 | Anti-FLT3LG antibody(DMC421); IgG1 Chimeric mAb |
| DMC101110 | Anti-FLT3 antibody(19A5), IgG1 Chimeric mAb | |
| PE-conjugated mAb | DMC101110P | PE-conjugated Anti-FLT3 antibody(19A5), IgG1 Chimeric mAb |
| DMC100421P | PE-conjugated Anti-FLT3LG antibody(DMC421); IgG1 Chimeric mAb | |
| Biotinylated mAb | DMC100421B | Biotinylated Anti-FLT3LG antibody(DMC421); IgG1 Chimeric mAb |
| DMC101110B | Biotinylated Anti-FLT3 antibody(19A5), IgG1 Chimeric mAb |
- Progress in FLT3 Lead Molecule Discovery

References:
- Annesley, C.E. and P. Brown, The Biology and Targeting of FLT3 in Pediatric Leukemia. Front Oncol, 2014. 4: p. 263.
- Naoe, T. and H. Kiyoi, Normal and oncogenic FLT3. Cell Mol Life Sci, 2004. 61(23): p. 2932-8.
- Kazi, J.U. and L. Ronnstrand, FMS-like Tyrosine Kinase 3/FLT3: From Basic Science to Clinical Implications. Physiol Rev, 2019. 99(3): p. 1433-1466.
- Daver, N., et al., Targeting FLT3 mutations in AML: review of current knowledge and evidence. Leukemia, 2019. 33(2): p. 299-312.
- Zhao, J.C., et al., A review of FLT3 inhibitors in acute myeloid leukemia. Blood Rev, 2022. 52: p. 100905.