- Protein purity detection
Proteins for solid tumor targets
In recent years, the biopharmaceutical market is growing rapidly. Compared with small molecule drugs, antibody drugs exhibit higher specificity and selectivity. Therefore, it showed tremendous progresses in the treatment of hematological cancers and autoimmune diseases. Due to complex tumor immune microenvironment, the development of therapeutic antibody drugs for solid tumor is more challenging.
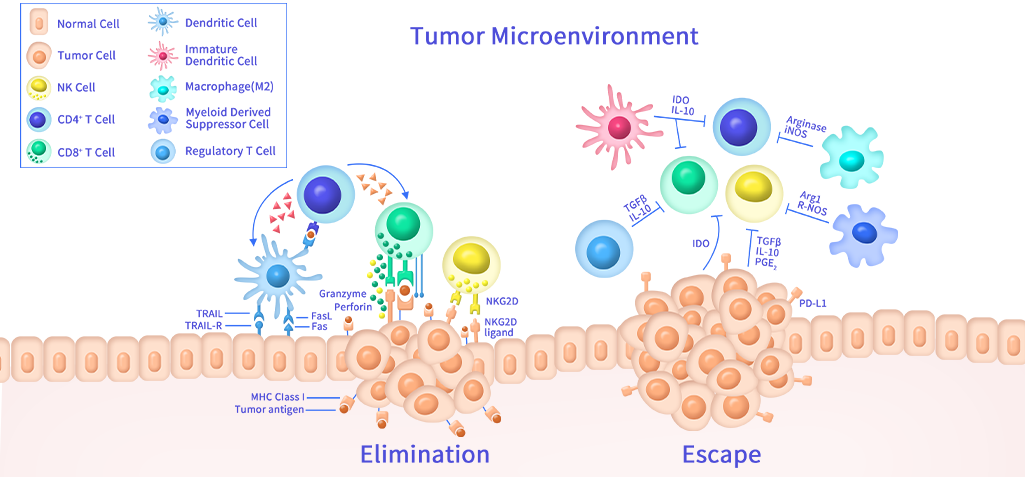
Solid tumors account for the majority of global cancer incidence and remain the primary focus of antibody, ADC, and cell therapy development. Target discovery efforts increasingly center on tumor-specific surface antigens, microenvironment-associated proteins, and oncogenic driver alterations. Therapeutic antibodies need to exert their functional activities in human body. Therefore, it is very critical to identify monoclonal antibodies with functional activities. “Garbage in Garbage out” is the rule of thumb for therapeutic antibody development.
DIMA BIOTECH pays special attention on its immunogen development process. All the proteins were made by using HEK293 mammalian cell secretion expression system and we implemented a strict quality control process, including purity testing, antibody-drug interaction verification, freezing and thawing Tests, thermal stability tests, etc.
Core Surface Targets in Solid Tumors
Tumor-associated membrane proteins are leading targets for monoclonal antibodies, antibody-drug conjugates (ADCs), bispecific antibodies, and CAR-T therapies. Below are representative high-interest solid tumor targets:
Claudin 18.2 (CLDN18.2)
A tight junction protein selectively expressed in gastric and pancreatic cancers. A prominent target in ADC and CAR-T development.
Mesothelin (MSLN)
Overexpressed in mesothelioma, ovarian cancer, and pancreatic cancer. Widely pursued in antibody and cell therapy programs.
TROP2
A transmembrane glycoprotein broadly expressed across epithelial cancers.
Explore the latest progress of ADC targeting TROP2HER2
HER2 is a common molecular target in various cancers, especially in breast cancer and gastric cancer.
Explore the latest progress of drugs targeting HER2Nectin-4
A cell adhesion molecule highly expressed in urothelial carcinoma and other epithelial tumors.
Explore the latest progress of drugs targeting Nectin-4AXL
Associated with tumor invasion, metastasis, and therapy resistance across multiple solid tumor types.
Explore the latest progress of drugs targeting AXLApplications
- Immunogens for antibody drug development
- Reagents forIn vitro orin vivo functional assays
- Reagents to be used for drug lead molecule validation
- Antibody affinity measurement assays
Strict Quality Control
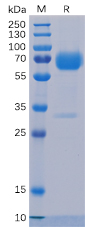
Figure 1. Human Trop2 Protein, mFc-His Tag on SDS-PAGE under reducing condition.
- Protein purity detection
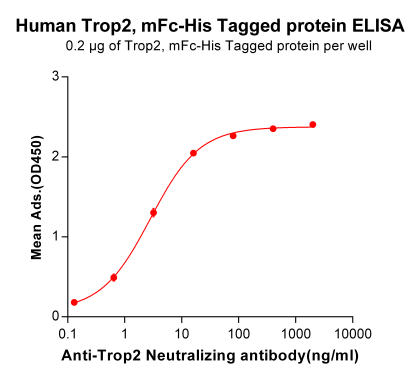
Figure 2. Immoblized Human Trop2 protein (PME100501) can bind Anti-Trop2 Neutralizing antibody (BME100023) in a linear range of 0.13-16.0 ng/ml.
Targets
| ALK | AXL | CEACAM5 | CLDN18.2 | CLDN6 | EGFR | EPCAM | FAP |
| FGFR2 | FGFR3 | HER2 | KRAS | MET | MSLN | MUC1 | NECTIN4 |
| NTRK1 | PSCA | PTK7 | RET | ROR1 | ROR2 | TGFBR2 | TROP2 |
| VEGFA | YAP1 |
