In recent years, immunotherapy targeting the PD-1/PD-L1 axis has undergone rapid evolution. The field has moved far beyond first-generation single-target monoclonal antibodies, expanding into highly competitive modalities such as PD-1/VEGF bispecific antibodies, PD-L1 antibody drug conjugates (ADCs), and a wide range of combination immunotherapy strategies. Both clinical development and capital markets are clearly shifting upstream.
Landmark transactions such as SSGJ-707 have not only reaffirmed the commercial value of immune checkpoint inhibitors, but also highlighted a critical industry insight: true differentiation and long-term ceiling are determined not by whether to pursue immuno-oncology, but by which target to enter and how to enter it.
So, within the same PD-1/PD-L1 immune axis, what do PD-1 targeted and PD-L1 targeted strategies actually mean in terms of biological rationale and clinical decision-making? To answer this, we must begin with the signaling pathway itself.
1. Overview of the PD-1/PD-L1 Signaling Mechanism
Programmed Death Receptor 1 (PD-1) is an inhibitory receptor expressed on activated T cells. Its ligand, Programmed Death Ligand 1 (PD-L1), is expressed on tumor cells as well as antigen-presenting cells (APCs).
Within the tumor microenvironment (TME), binding of PD-L1 to PD-1 triggers downstream inhibitory signaling cascades that lead to T-cell exhaustion, reduced cytotoxic activity, and ultimately immune escape by tumor cells. Blocking the interaction between PD-1 and PD-L1 restores T-cell proliferation and effector function, thereby reactivating or amplifying antitumor immune responses.
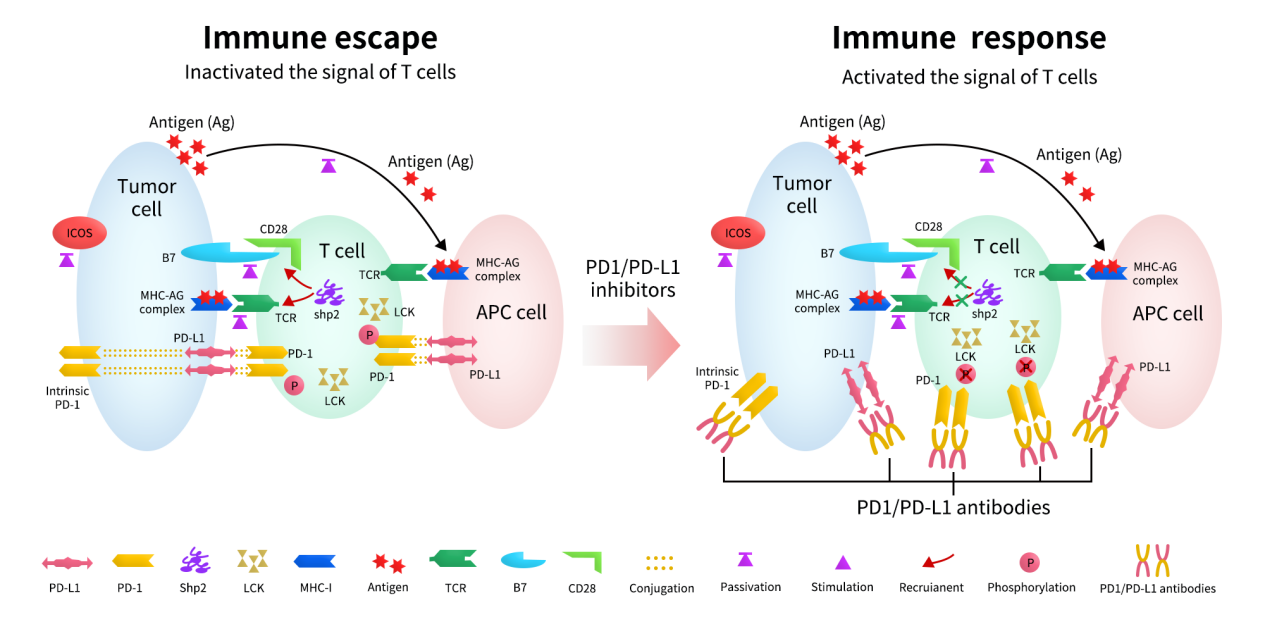
Figure 1. Mechanisms of the response to anti-PD1/PD-L1 immunotherapy [1]
Explore more information of PD-1/PD-L1 Signaling pathway
2.Targeting PD-1 vs Targeting PD-L1: Key Differences and Strategic Implications
Targeting the PD-1/PD-L1 pathway has become a cornerstone of modern cancer immunotherapy. Current strategies include PD-1 inhibitors, PD-L1 inhibitors, and a growing number of combination and next-generation approaches. So which is better: PD-1 or PD-L1? The prevailing industry consensus is: PD-1 targeted therapies tend to show broader and deeper clinical efficacy; PD-L1 targeted therapies offer advantages in safety, combination flexibility, and innovative drug design.
2.1 Mechanism of Action: PD-1 Blockade Is More Comprehensive
PD-1 is located on the surface of T cells. Targeting PD-1 blocks not only the interaction between PD-1 and PD-L1, but also between PD-1 and PD-L2. PD-L2 is expressed at meaningful levels in certain tumors and inflammatory tissues (such as lung and head & neck cancers). As a result, PD-1 inhibition more fully releases T-cell suppression, which is widely regarded as a key biological reason for its slightly superior efficacy.
By contrast, PD-L1 targeted therapies are more selective. PD-L1 is primarily expressed on tumor cells and APCs. Blocking PD-L1 inhibits its interaction with PD-1 and B7-1 (CD80), but does not interfere with PD-1/PD-L2 signaling.
2.2 Clinical Efficacy: PD-1 Remains the Mainstream Choice
Comparisons between PD-1 inhibitors (such as Keytruda®, Opdivo®, Hansizhuang®) and PD-L1 inhibitors (such as Tecentriq®, Imfinzi®, Bavencio®) consistently show that PD-1 therapies lead in:
- Number of approved indications
- Objective response rate (ORR) and overall survival (OS)
- Share of first-line treatment
- Pan-cancer applicability
In core indications such as NSCLC, melanoma, and gastric cancer, PD-1 inhibitors are more frequently adopted as first-line therapies.
2.3 Safety Profile: PD-L1 Is Often Undervalued
One commonly overlooked advantage of PD-L1 targeting is improved safety. PD-1 inhibitors act broadly on all T cells, whereas PD-L1 inhibitors exert effects more locally within the tumor and immune microenvironment.
As a result, PD-L1 inhibitors are associated with slightly lower rates of immune-related adverse events (irAEs), particularly pneumonitis, thyroiditis, and colitis. This safety advantage helps explain the strong clinical positioning of Imfinzi® (durvalumab) in consolidation therapy following chemoradiation.
2.4 Combination and Innovation Potential: PD-L1 Is Gaining Momentum
Because PD-L1 is expressed on the surface of tumor cells, it serves as a more suitable “anchoring target” for structural drug design, making it highly attractive for innovation.
Consequently, next-generation formats tend to favor PD-L1, including: PD-L1xTGF-β bispecific antibodies and PD-L1xTIGIT bispecific antibodies. While PD-1 based bispecifics such as PD-1xVEGF or PD-1xCTLA-4 do exist, they are fewer in number compared with PD-L1 based combinations.
Moreover, PD-L1 is better suited for ADCs and delivery-based therapeutics due to its tumor cell surface expression and favorable internalization properties. Nearly all ADCs and immune-stimulatory conjugates targeting the PD-1/PD-L1 axis currently focus on PD-L1 rather than PD-1.
3.Why Does PD-1 Still Dominate If PD-L1 Offers Better Safety and Innovation Potential?
The answer lies largely in clinical familiarity and execution risk. PD-1 inhibitors are highly trusted by clinicians, cover broad indications, require lower market education costs, and follow well-established development pathways, particularly in China. For many companies, developing a differentiated PD-1 drug represents a lower-risk strategy than pioneering a novel PD-L1 program.
That said, PD-L1 provides a clear path away from the crowded PD-1 red ocean. It is better positioned for bispecific antibodies, ADCs, and combination regimens involving chemotherapy, radiotherapy, or neoadjuvant settings, offering greater long-term innovation upside.
Ultimately, the choice depends on a company’s strategic priorities:
- If the goal is higher clinical success probability and rapid commercialization, PD-1 may be preferable.
- If the objective is long-term differentiation through innovative modalities, PD-L1 holds greater potential.
Explore more information of the latest clinical progress of drugs targeting PD-1
Explore more information of the latest clinical progress of drugs targeting PD-L1

