Autoimmune diseases are a diverse and complex group of disorders characterized by immune dysregulation resulting from aberrant interactions between the innate and adaptive immune systems. Their pathogenesis is multifactorial, and the clinical manifestations are highly heterogeneous, posing significant challenges to effective treatment. Although recent advances have shed light on key pathological processes and molecular mechanisms, many obstacles remain in clinical management.
One of the most notable features of autoimmune diseases is the presence of autoantibodies, which are produced by autoreactive B cells. These aberrant antibodies target the body’s own cells and tissues, triggering inflammation and organ damage. Current therapeutic strategies mainly include immunosuppressants, glucocorticoids, and biologics.
CD20, a transmembrane glycoprotein primarily expressed on the surface of B lymphocytes, has emerged as a critical therapeutic target. Multiple studies have demonstrated that abnormal B cells play a central role in the pathogenesis of various autoimmune diseases, making CD20 an important target not only in hematologic malignancies but also in certain autoimmune conditions.
1. Structure of CD20
CD20 (B-lymphocyte surface antigen) is a 33–37 kDa non-glycosylated protein that belongs to the MS4A (membrane-spanning 4-domains subfamily A) protein family. In humans, CD20 is encoded by the MS4A1 gene located on chromosome 11q12.2. The CD20 molecule is a 297-amino acid phosphoprotein with four transmembrane domains (Fig. 1), and it plays a critical role in B cell development and activation.
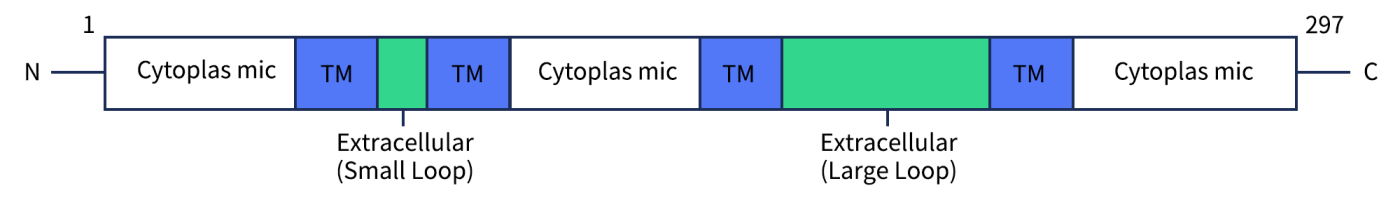
Fig 1. The structure of CD20.[1]
2. CD20 Expression
CD20 is a transmembrane glycoprotein expressed on the surface of B lymphocytes. Under physiological conditions, CD20 is present during the pre-B cell and mature B cell stages, but its expression is lost once B cells differentiate into plasma cells. Under pathological conditions, CD20 is highly expressed in hematological malignancies of B cell origin, such as B-cell lymphomas and leukemias.
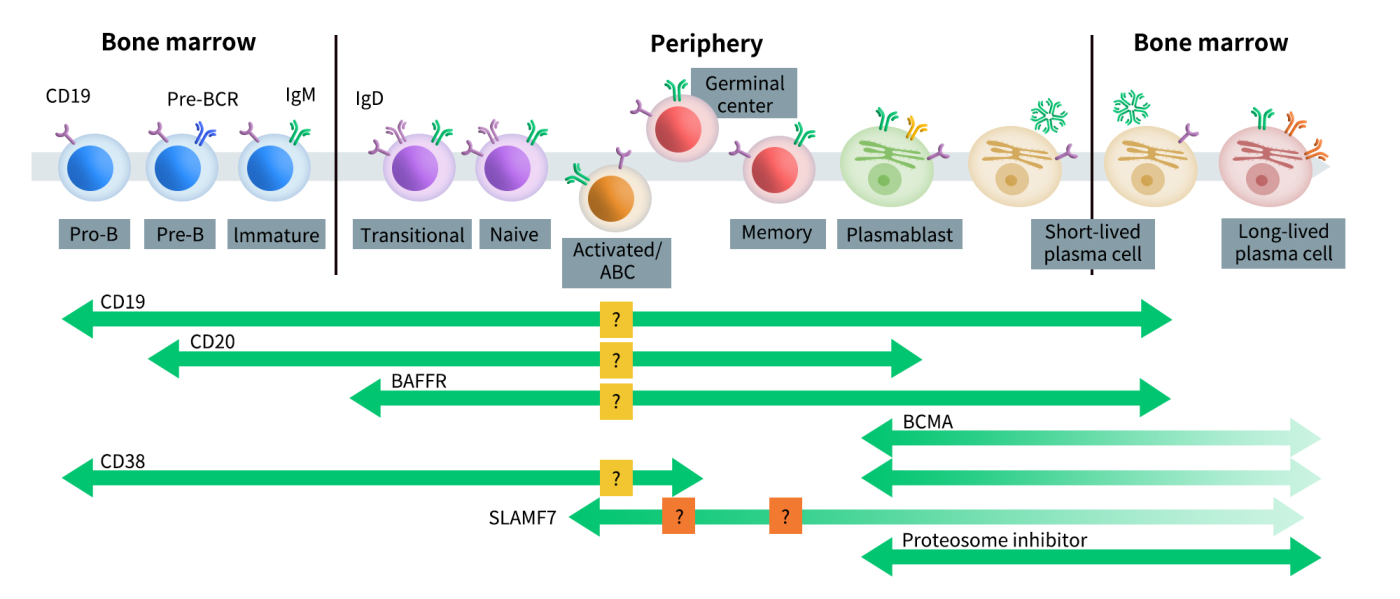
Fig 2. B cell developmental pathways and therapeutic targets.[2]
3. Role of CD20 in Autoimmune Diseases
Autoimmune diseases arise from dysfunction of the immune system, in which immune cells mistakenly attack the body’s own tissues, leading to systemic diseases or localized damage, such as systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA). In the pathogenesis of various autoimmune diseases, autoreactive B cells play a pivotal role. A large number of preclinical and clinical studies have demonstrated that B cell depletion using anti-CD20 monoclonal antibodies (mAbs) can effectively slow disease progression.
Although CD20 does not directly participate in antigen recognition or antibody production during immune responses, it plays a crucial role in B cell activation, differentiation, proliferation, and calcium ion channel regulation. As such, therapeutic strategies targeting CD20 to deplete B cells have been widely applied in the treatment of several autoimmune diseases.
Below is an overview of the functions and roles of CD20 in major autoimmune diseases:
3.1 Multiple Sclerosis (MS)
Multiple sclerosis is a chronic autoimmune demyelinating disease of the central nervous system, traditionally considered to be mainly T-cell-mediated. However, recent studies have shown that B cells also play a key role in MS, evidenced by the presence of myelin antigen-specific antibodies in the cerebrospinal fluid and the formation of ectopic B cell follicle-like structures. CD20⁺ B cells may enhance T cell responses by presenting antigens, expressing co-stimulatory molecules, and secreting pro-inflammatory cytokines such as IL-6 and TNF-α, thereby exacerbating neuroinflammation.
3.2 Rheumatoid Arthritis (RA)
Rheumatoid arthritis is a chronic inflammatory joint disease in which B cells contribute to inflammation and joint destruction through the production of autoantibodies (e.g., anti-CCP, rheumatoid factor), activation of T cells, and secretion of pro-inflammatory cytokines. CD20⁺ B cells are enriched in synovial tissues and peripheral blood, driving both local and systemic inflammatory responses.
3.3 Systemic Lupus Erythematosus (SLE)
Systemic lupus erythematosus is a systemic autoimmune disease characterized by abnormal B cell activation and the production of pathogenic autoantibodies. Although the clinical efficacy of rituximab (anti-CD20 mAb) in SLE has been variable, it has shown significant benefit in patients with refractory lupus nephritis or hematological involvement.
3.4 Psoriasis and Psoriatic Arthritis (PsA)
Psoriasis is a chronic immune-mediated disease characterized by abnormal keratinocyte proliferation and immune dysregulation, while PsA involves joint inflammation. Although the role of B cells in the pathogenesis of psoriasis and PsA is less well-defined compared to RA or MS, abnormal B cell activation has been observed in some PsA patients. Elevated expression of CD20⁺ B cells has been detected in the synovial tissue of PsA patients, potentially contributing to disease pathogenesis through antigen presentation and activation of Th17 cells. Anti-CD20 therapy is still in the early research stage for psoriasis, with no approved drugs yet, but it offers a potential new strategy for treatment-refractory patients.
3.5 Sjögren’s Syndrome (SS)
Sjögren’s syndrome is an autoimmune disease characterized by damage to exocrine glands, leading to symptoms such as dry mouth and dry eyes. B cells infiltrate the salivary and lacrimal glands and produce autoantibodies such as anti-SSA/Ro and anti-SSB/La. CD20⁺ B cells show abnormal proliferation and activation in both peripheral blood and glandular tissues of patients, correlating with disease activity and an increased risk of malignant transformation, such as MALT lymphoma.
4. Therapeutic Advances of CD20 Targeting in Autoimmune Diseases
Targeted therapies against CD20 have seen expanding applications in autoimmune diseases. These include classical monoclonal antibodies such as rituximab (approved for RA, SLE, etc.), engineered antibodies like obinutuzumab (enhanced ADCC activity) and ofatumumab (fully humanized, approved for MS), as well as emerging bispecific antibodies such as ocrelizumab and veltuzumab, which exhibit improved B cell depletion. In addition, several rituximab biosimilars have entered the market, increasing accessibility and treatment options for patients. By targeting B cells, these therapies have demonstrated efficacy in multiple B cell-mediated diseases, including RA, SLE, and MS.
4.1 Multiple Sclerosis (MS)
CD20-targeted therapy has achieved major breakthroughs in MS and is now a standard strategy for both relapsing and progressive forms of the disease.
- Ocrelizumab is the first anti-CD20 monoclonal antibody approved for MS. It is a humanized IgG1 antibody with potent B cell-depleting capacity. Approved by the FDA and EMA in 2017, it is indicated for relapsing-remitting MS (RRMS) and primary progressive MS (PPMS) — the only drug currently approved for PPMS. In pivotal Phase III trials (OPERA I/II and ORATORIO), ocrelizumab significantly reduced annual relapse rates, MRI lesion burden, and the progression of disability.
- Ofatumumab, a fully human anti-CD20 mAb that binds to a membrane-proximal epitope on CD20, effectively induces complement-dependent cytotoxicity (CDC). It is administered via subcutaneous injection, allowing patients to self-administer at home. Approved by the FDA in 2020 for relapsing MS (RMS), Phase III ASCLEPIOS trials showed comparable efficacy to ocrelizumab with a potentially lower risk of infections.
- Rituximab, although not formally approved for MS, is widely used off-label, particularly in Nordic countries. Studies show it significantly reduces MS relapses, MRI lesion activity, and slows disability progression. However, being a chimeric antibody, rituximab carries risks of immunogenicity and allergic reactions with long-term use.
- Ublituximab (TG-1101), a second-generation glycoengineered anti-CD20 mAb with reduced fucosylation to enhance ADCC, has shown positive results in the Phase III ULTIMATE I/II trials and is currently under regulatory review for MS treatment.
4.2 Rheumatoid Arthritis (RA)
CD20-targeting therapy is well-established in RA.
- Rituximab remains the only anti-CD20 antibody approved for RA (FDA approval in 2006). It is indicated for moderately to severely active RA in patients who have had an inadequate response to one or more TNF-α inhibitors and is used in combination with methotrexate. Rituximab effectively depletes B cells in circulation and synovial fluid, reduces autoantibody levels (e.g., RF, ACPA), and slows joint destruction. Phase III trials such as REFLEX and MIRROR confirmed its clinical benefit in improving disease activity and quality of life.
- Ocrelizumab and ofatumumab were evaluated in early RA trials (e.g., SCRIPT for ocrelizumab), but development for this indication was discontinued due to strategic and efficacy considerations.
- Next-generation antibodies like obinutuzumab and veltuzumab are still under investigation for RA, with no advanced-stage clinical data available yet.
4.3 Systemic Lupus Erythematosus (SLE)
Given the central role of B cells in SLE pathogenesis, CD20-targeted therapies have been extensively explored.
- Rituximab was among the first CD20 antibodies studied in SLE. However, it failed to meet primary endpoints in pivotal Phase III trials (EXPLORER and LUNAR), which prevented its formal approval. Nonetheless, it is commonly used off-label in clinical practice, especially for refractory cases or organ-threatening manifestations such as lupus nephritis and neuropsychiatric lupus.
- To overcome the limitations of rituximab, more potent CD20 antibodies have been developed. Obinutuzumab, a type II humanized anti-CD20 mAb, exhibits stronger B cell depletion and less internalization than rituximab. In the Phase III NOBILITY trial, it showed promising results in improving proteinuria and kidney function in lupus nephritis patients, and further confirmatory studies are ongoing.
- Inebilizumab, an anti-CD19 antibody that targets a broader range of B cell subsets including plasmablasts, is being investigated as an alternative in SLE and other B cell-driven autoimmune diseases, potentially offering expanded therapeutic benefits.
4.4 Sjögren’s Syndrome (SS)
B cell activation plays a critical role in SS pathogenesis.
- Rituximab is the most commonly used anti-CD20 mAb in SS, although it has not received regulatory approval. Several small-scale clinical trials (e.g., TEARS, TRIPSS) have shown partial improvements in symptoms such as dryness, fatigue, and salivary gland function. However, inconsistent efficacy and failure to meet primary endpoints have prevented guideline endorsement.
- Newer CD20 antibodies such as obinutuzumab and veltuzumab are under investigation in animal models and early-phase clinical studies. These agents appear to have better tissue penetration and B cell-depleting efficacy in target organs like salivary and lacrimal glands.
- Recent studies have also begun exploring the role of CD20⁺ T cells in SS, opening up new avenues for therapeutic intervention.
5. CD20 Target-Related Products
DIMA now offers a range of in-stock products targeting CD20, including recombinant proteins, full-length membrane proteins, recombinant monoclonal antibodies, and biotin/PE-labeled antibodies. We also provide comprehensive services such as protein/antibody customization, antibody humanization, affinity maturation, and stable cell line development.
In addition, we have established a B cell seed library for the CD20 target, enabling rapid screening of lead antibody candidates within just 20 days.
- CD20-Related Product Catalog
- Partial Data Display
① Recombinant Protein
Human CD20 Protein, hFc Tag(Cat. No.PME100046)
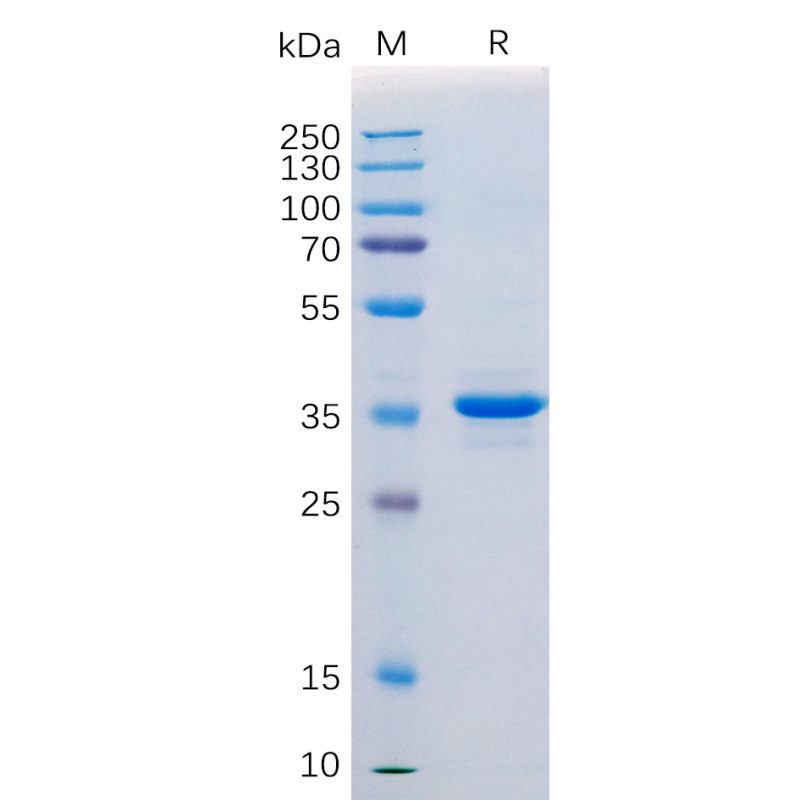
Human CD20 Protein, hFc Tag on SDS-PAGE.
② Full-Length Membrane Protein
Human CD20 full length protein-MNP(Cat.No.FLP100026)
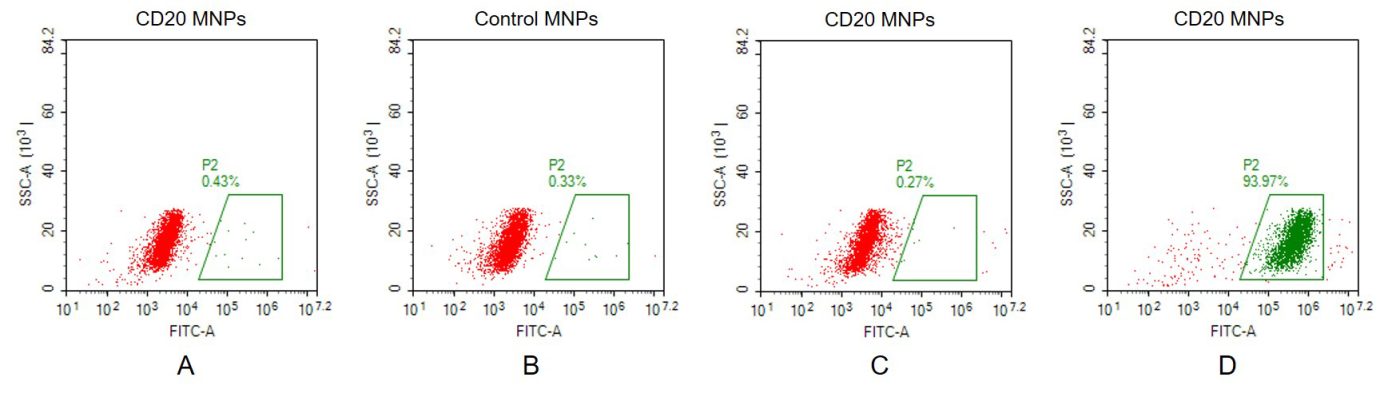
A. Negative Control 1: CD20 full length membrane nanoparticles samples were stained only with Goat anti-human lgG 488 secondary antibody; B. Negative Control 2: Control membrane nanoparticles samples were stained with anti-CD20 antibody (BME100160) at 2μg/mL, followed by Goat anti-human IgG 488 secondary antibody; C. Negative Control 3: CD20 full length membrane nanoparticles samples were stained with anti-CCR8 antibody (an irrelevant antibody) at 2μg/mL, followed by Goat anti-human IgG 488 secondary antibody.; D. CD20 full length membrane nanoparticles samples were stained with anti-CD20 antibody (BME100160) at 2μg/mL, followed by Goat anti-human IgG 488 secondary antibody.
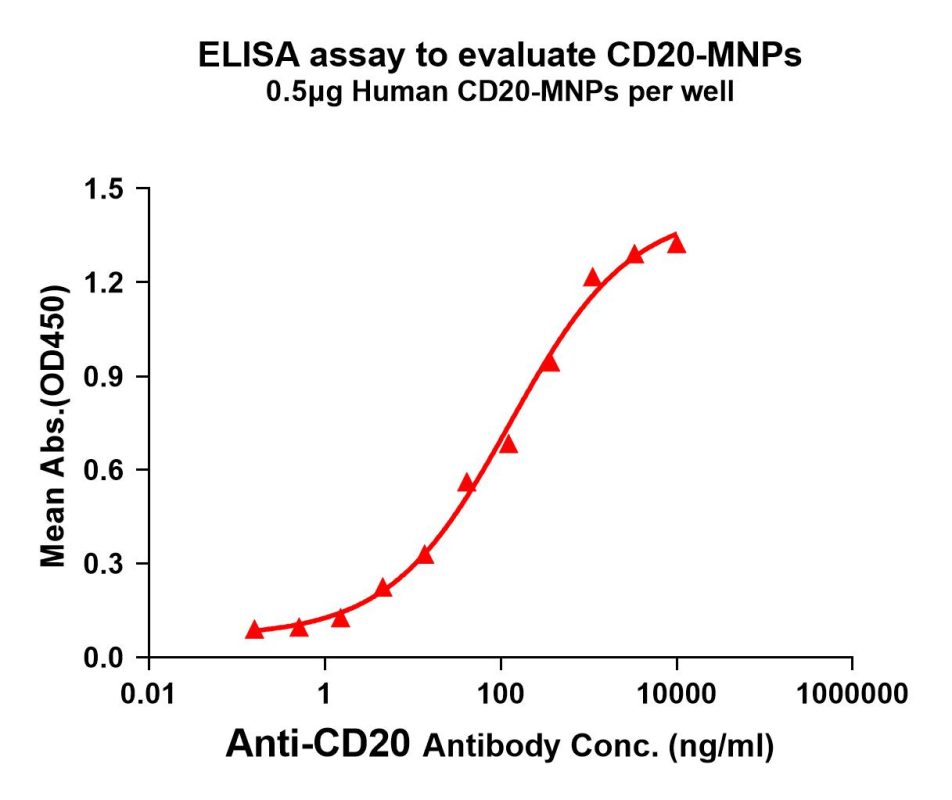
CD20-MNP can bind with anti-CD20 monoclonal antibody (BME100160) and the EC50 is 128.8ng/ml.
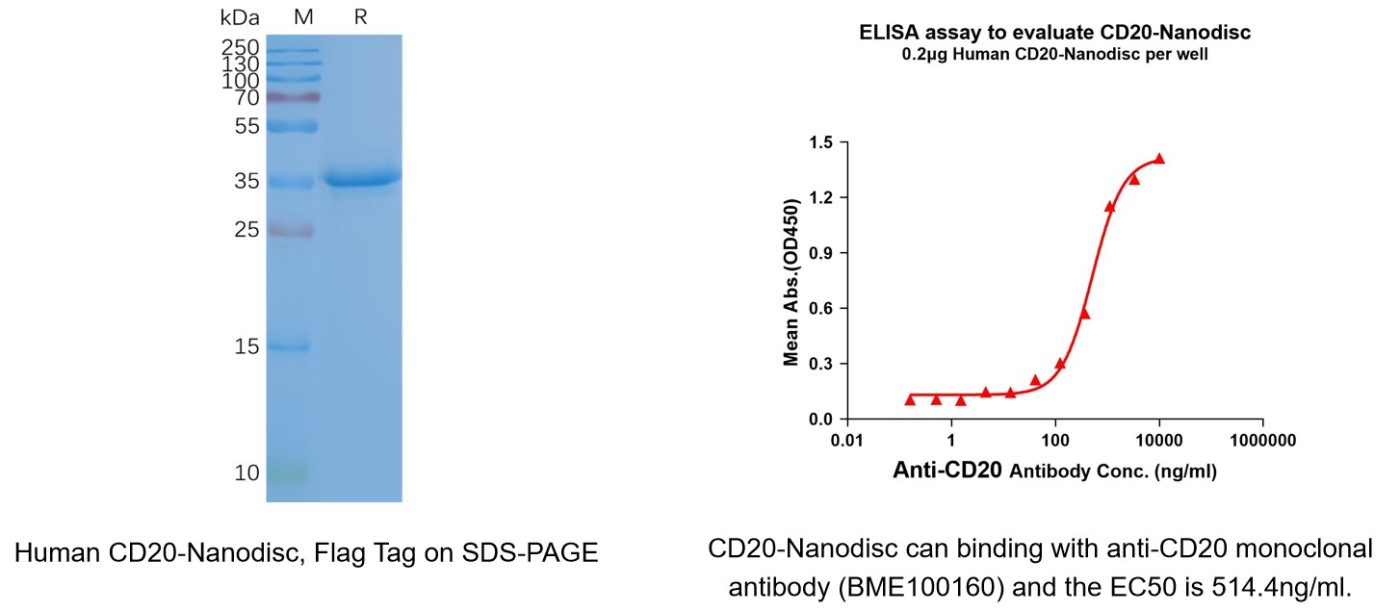
Human CD20 full length protein-synthetic nanodisc(Cat. No.FLP100027)
③ Recombinant Monoclonal Antibody
Anti-CD20 antibody(1F2), IgG1 Chimeric mAb(Cat. No.DMC101229)
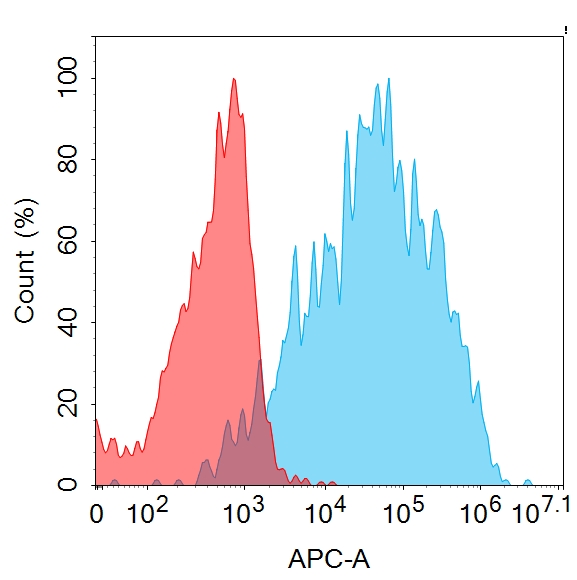
Flow cytometry analysis with 1μg/mL Anti-CD20 (1F2) mAb on HEK293 cells transfected with human CD20 (Blue histogram) or HEK293 transfected with irrelevant protein (Red histogram).
④ Biosimilar Reference Antibody
Anti-CD20 (obinutuzumab biosimilar) mAb(Cat. No.BME100160)
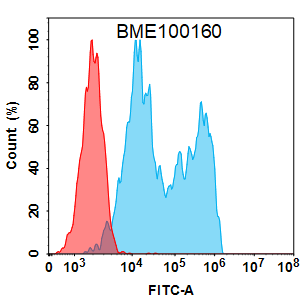
Flow cytometry analysis with 2 μg/mL Anti-CD20 (obinutuzumab biosimilar) mAb (BME100160) on HEK293 cells transfected with Human CD20 protein (Blue histogram) or HEK293 transfected with irrelevant protein (Red histogram).
Anti-CD20 (rituximab biosimilar) mAb(Cat. No.BME100025)
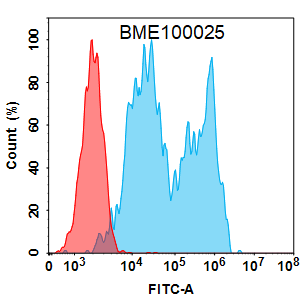
Flow cytometry analysis with 2 μg/mL Anti-CD20 (rituximab biosimilar) mAb (BME100025) on HEK293 cells transfected with Human CD20 protein (Blue histogram) or HEK293 transfected with irrelevant protein (Red histogram).
- Progress of Lead Antibody Candidates Targeting CD20

Reference
1. Cang, S., et al., Novel CD20 monoclonal antibodies for lymphoma therapy. J Hematol Oncol, 2012. 5: p. 64.
2. Abeles, I., et al., B Cell-Directed Therapy in Autoimmunity. Annu Rev Immunol, 2024. 42(1): p. 103-126.
3. Hauser, S.L., et al., Ocrelizumab versus Interferon Beta-1a in Relapsing Multiple Sclerosis. N Engl J Med, 2017. 376(3): p. 221-234.
4. Edwards, J.C., et al., Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med, 2004. 350(25): p. 2572-81.
5. Lee, A.Y.S., CD20(+) T cells: an emerging T cell subset in human pathology. Inflamm Res, 2022. 71(10-11): p. 1181-1189.
6. Devauchelle-Pensec, V., et al., Treatment of primary Sjogren syndrome with rituximab: a randomized trial. Ann Intern Med, 2014. 160(4): p. 233-42.
