In recent years, nanobodies (single-domain antibodies, VHHs) have rapidly emerged as a hot research direction in antibody and biologics development, thanks to their unique structure and biological properties. Derived from camelids, these single-domain antibodies feature a small size, high stability, and easy engineering capabilities, making them ideal for applications in drug discovery, diagnostics, imaging, and immunotherapy.
However, despite their advantages, many R&D teams still face long development cycles, slow project initiation, and challenges identifying high-affinity nanobody candidates. This is where DIMA BIOTECH’s stock-ready nanobody platform provides a significant breakthrough—allowing scientists to streamline early discovery and accelerate their therapeutic pipeline with minimal risk.
1. Discovery of Nanobodies: The Origin of Single-Domain Antibodies
The discovery of nanobodies (VHH, single-domain antibodies) began in 1993, when scientists identified a unique class of antibodies in camelid serum, structurally distinct from conventional IgG. These antibodies consist of heavy chains only, lacking light chains, yet they still specifically bind antigens [1].
This finding challenged the long-standing belief that functional antigen-binding requires both heavy and light chains. Camelid heavy-chain antibodies demonstrated that a single variable heavy-chain domain (VHH) can independently recognize antigens.
Subsequent studies confirmed the presence of these natural heavy-chain antibodies in llamas, alpacas, and other camelids, and established methods to obtain VHHs from immunized animals. This discovery laid the foundation for nanobody production and engineering, opening the door for systematic research from basic immunology to drug development.
2. Why Nanobodies Are Reshaping Modern Biologics
Nanobodies are extremely compact, typically 12–15 kDa, roughly ten times smaller than conventional IgG antibodies. Their small size facilitates tissue penetration, including tumor microenvironments, enhancing their versatility and engineering potential.
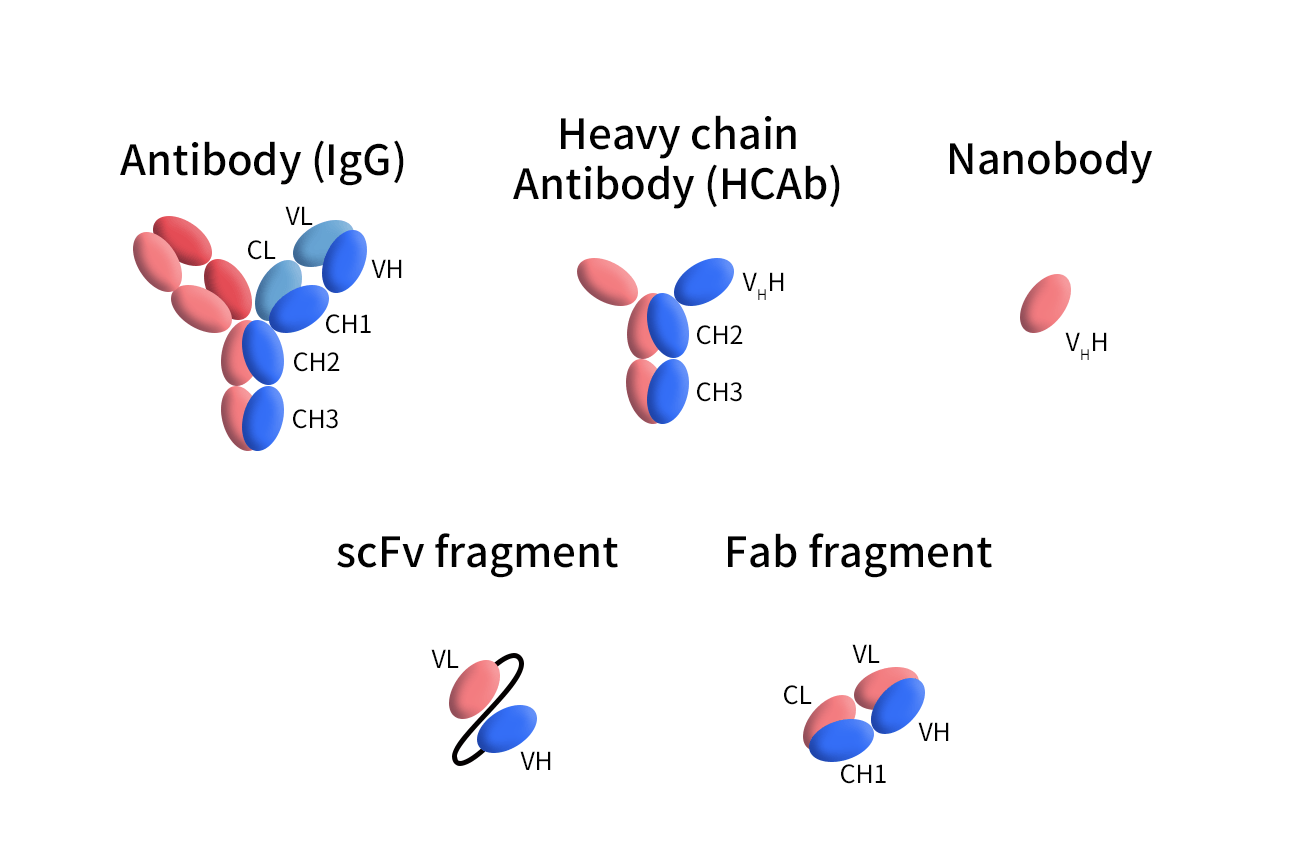
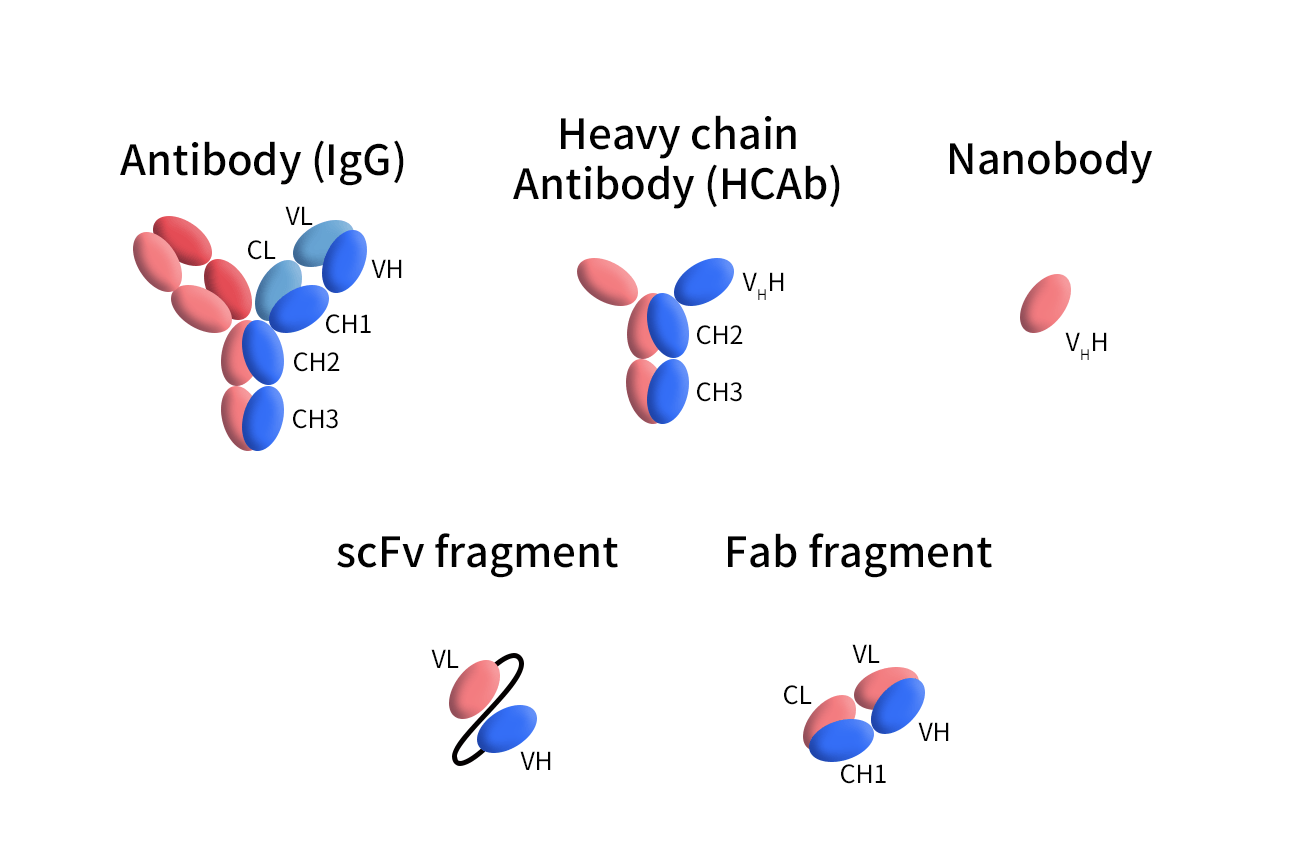
Figure 1. The Structures of different Antibodies
Nanobodies offer several structural and functional properties that make them ideal for next-generation biologics:
- Small, stable single-domain structure: Their compact VHH framework provides exceptional solubility and thermostability, enabling robust performance across diverse biochemical conditions (such as high temperatures, extreme pH, or organic solvents).
- High affinity and specificity: Capable of targeting complex antigens, including “difficult-to-drug” membrane proteins, enzymes, and conformation-dependent epitopes.
- Easy expression and cost-effectiveness: Efficiently produced in E. coli, yeast (e.g., Pichia pastoris), and mammalian systems, supporting scalable production and batch consistency.
- Engineering-friendly: Easily converted into multivalent or multispecific formats, Nanobody-Fc fusions, and Nanobody-Drug Conjugates (NDCs).
- High tissue penetration: Excellent for tumor targeting and intracellular delivery applications.
These properties make nanobodies inherently suitable for drug delivery, imaging probes, and diagnostic reagents [2].
3. Nanobody Production and Screening
The discovery of nanobodies is just the beginning. To transform them into usable research tools or therapeutic molecules, efficient production and screening strategies are essential.
|
Method |
Principle |
Advantages |
Application |
|
Phage Display |
Display VHH libraries (from immunized animals or synthetic libraries) on phage surfaces and select high-affinity binders |
High enrichment, large library capacity, multiple selection rounds |
Drug candidate development, membrane protein targets, early validation |
|
Yeast Display |
Express VHH on yeast surfaces and select high-affinity clones via FACS [3] |
Maintains eukaryotic folding, suitable for conformation-sensitive antigens |
Membrane proteins, activity-based screening |
|
Single B-Cell Isolation |
Isolate antigen-specific B cells from immunized animals’ peripheral blood |
Fast access to high-specificity monoclonal VHHs without constructing large libraries |
High-specificity antibody discovery, rapid immune response |
|
Synthetic/Artificial Libraries |
Design random or directed VHH libraries based on known sequences without animal immunization [4] |
Rapid delivery, sequence optimization, suitable for toxic antigens |
Hard-to-immunize antigens, in vitro development, early validation |
After sequence acquisition, nanobodies can be expressed in:
- E. coli: Cost-effective, suitable for non-glycosylated fragments.
- Yeast (Pichia, Saccharomyces): Ideal for industrial-scale fermentation.
- Insect or mammalian cells: For complex modifications or native-like folding.
Optimized strategies now ensure high yield, solubility, and correct folding through signal peptide selection, chaperone co-expression, and optimized culture/fermentation conditions [5].
4. Applications of Nanobodies in Biologics
4.1 Therapeutic Applications
Nanobodies can function as independent therapeutic molecules (monomeric or multivalent) to antagonize or neutralize pathological proteins. For example, caplacizumab (Cablivi®), the first clinically approved nanobody drug, targets von Willebrand factor to treat acquired thrombotic thrombocytopenic purpura (aTTP), representing the translation from research to clinical success.
Therapeutic nanobodies can be designed as:
- Neutralizing antibodies
- Immunomodulatory antibodies
- Multivalent or multispecific antibodies
- Nanobody-Fc fusion proteins
4.2 Nanobody-Drug Conjugates & Targeted Delivery
Due to their small size, nanobodies efficiently penetrate solid tumors and deliver drugs or toxins into the tumor microenvironment. They can be conjugated to toxins or small molecules to form Nanobody-Drug Conjugates (NDCs), or combined with liposomes/nanoparticles for RNA, siRNA, or protein delivery, enhancing efficiency and specificity. Their stability also supports complex administration routes (e.g., inhalation or local delivery).
4.3 Imaging and Radiotracer Applications
Nanobody VHHs rapidly clear from background and reach targets quickly, enabling high-contrast imaging in PET, SPECT, or fluorescence applications. Compared to conventional antibodies, nanobodies offer shorter imaging windows and higher sensitivity, suitable for clinical imaging and early diagnostic applications.
4.4 Other Applications
Construction of bispecific/multispecific molecules, targeting both tumor antigens and immune cell pathways, supports next-generation immunotherapy. In in vitro diagnostics (IVD) and rapid test reagents, nanobodies’ high stability ensures batch consistency, long shelf-life, and robust performance in ELISA, CLIA, virus detection, environmental and food assays.
5 The Challenge: Long Development Cycles Slow Down Your Pipeline
While nanobodies offer great promise, generating high-quality candidates often requires:
- complex immunization
- specialized screening technologies
- high-throughput affinity selection
- iterative optimization steps
These processes can take months—delaying early experiments and slowing target validation, lead identification, and mechanism studies. Many teams simply need a high-quality nanobody “right now” to initiate assays, confirm target engagement, or kickstart projects.
This is exactly why DIMA BIOTECH developed its Stock-Ready Nanobody Platform.
5. DIMA BIOTECH's Stock-Ready Nanobody Platform: Zero Cycle, Zero Upfront Cost, Zero Risk
To meet the growing demand, DIMA BIOTECH offers a full range of nanobody custom services covering antigen design, immunization or in vitro library screening, sequence engineering, expression optimization, and scale-up production.
To help researchers accelerate timelines and reduce uncertainty, DIMA BIOTECH offers Off-the-shelf, application-validated nanobodies targeting key molecules in oncology, immunology, viral research, and especially membrane protein targets. Customers can immediately test sequences without waiting for weeks or months for immunization and screening, significantly reducing early-stage project timelines and costs.
Platform Advantages:
- Zero Waiting: Obtain and validate sequences on the same day.
- Zero Upfront: Test first, pay later, only if sequences meet expectations.
- Zero Risk: Avoid uncertainty from unusable immunizations, improving success rates.
Validated sequences can further undergo affinity maturation or sequence optimization for preclinical or mechanistic studies. This approach accelerates concept-to-molecule translation while minimizing cost and risk.
Reference:
- [1]Hamers-Casterman, C., Atarhouch, T., Muyldermans, S. et al. Naturally occurring antibodies devoid of light chains. Nature 363, 446–448 (1993).
- [2]Muyldermans S. Nanobodies: natural single-domain antibodies. Annu Rev Biochem. 2013;82:775-97.
- [3]McMahon C, Baier AS, Pascolutti R, Wegrecki M, Zheng S, Ong JX, Erlandson SC, Hilger D, Rasmussen SGF, Ring AM, Manglik A, Kruse AC. Yeast surface display platform for rapid discovery of conformationally selective nanobodies. Nat Struct Mol Biol. 2018 Mar;25(3):289-296.
- [4]Alexander, E., Leong, K.W. Discovery of nanobodies: a comprehensive review of their applications and potential over the past five years. J Nanobiotechnol 22, 661 (2024).
- [5]Zheng, Y.; Li, B.; Zhao, S.; Liu, J.; Li, D. A Universal Strategy for the Efficient Expression of Nanobodies in Pichia pastoris. Fermentation 2024, 10, 37.