On January 10, Harbour BioMed and Kelun-Biotech out-licensed the overseas rights of their jointly developed TSLP antibody HBM9378/SKB378 to Windward Bio. The deal reached a total value of up to USD 970 million. HBM9378/SKB378 is a fully human anti-TSLP monoclonal antibody co-developed by Harbour BioMed and Kelun-Biotech, designed to regulate immune responses by inhibiting the thymic stromal lymphopoietin (TSLP) signaling pathway for the treatment of immune-related diseases such as asthma and chronic obstructive pulmonary disease (COPD).
On September 15, CStone Pharmaceuticals announced that CS2015, one of its pipeline candidates in the field of autoimmune and inflammatory diseases, was selected for presentation at the 2025 Annual Scientific Meeting of the American College of Allergy, Asthma & Immunology (ACAAI). CS2015 is a bispecific antibody targeting both OX40L and TSLP. By simultaneously inhibiting key regulatory factors of Th2-mediated inflammatory responses, CS2015 offers a novel therapeutic strategy for type 2 inflammatory diseases such as atopic dermatitis (AD), asthma, and COPD.
TSLP is implicated in a variety of diseases, particularly playing a central role in allergic and inflammatory disorders such as asthma, atopic dermatitis, allergic rhinitis, and inflammatory bowel disease. Moreover, recent studies have revealed that TSLP also contributes to tumor immune evasion and autoimmune diseases, making it an attractive therapeutic target with broad potential applications. [1]
1. Structure of TSLP and Its Receptor Complex
Thymic Stromal Lymphopoietin (TSLP) is a type II cytokine secreted by epithelial cells. It was first isolated from a murine thymic stromal cell line and identified as a lymphocyte growth factor. [2]
- Molecular Structure
TSLP belongs to the four-α-helix bundle cytokine family and shares strong structural similarity with IL-7. In the human genome, the TSLP gene is located at chromosome 5q22.1. Its transcript encodes a protein of 159 amino acids, which is secreted as a glycoprotein with a molecular weight of approximately 15–18 kDa. Recent studies have identified two splice variants [3]:
Long-form TSLP: The classical isoform, involved in allergic and inflammatory responses.
Short-form TSLP: Its function remains incompletely understood but may be related to maintaining tissue homeostasis.
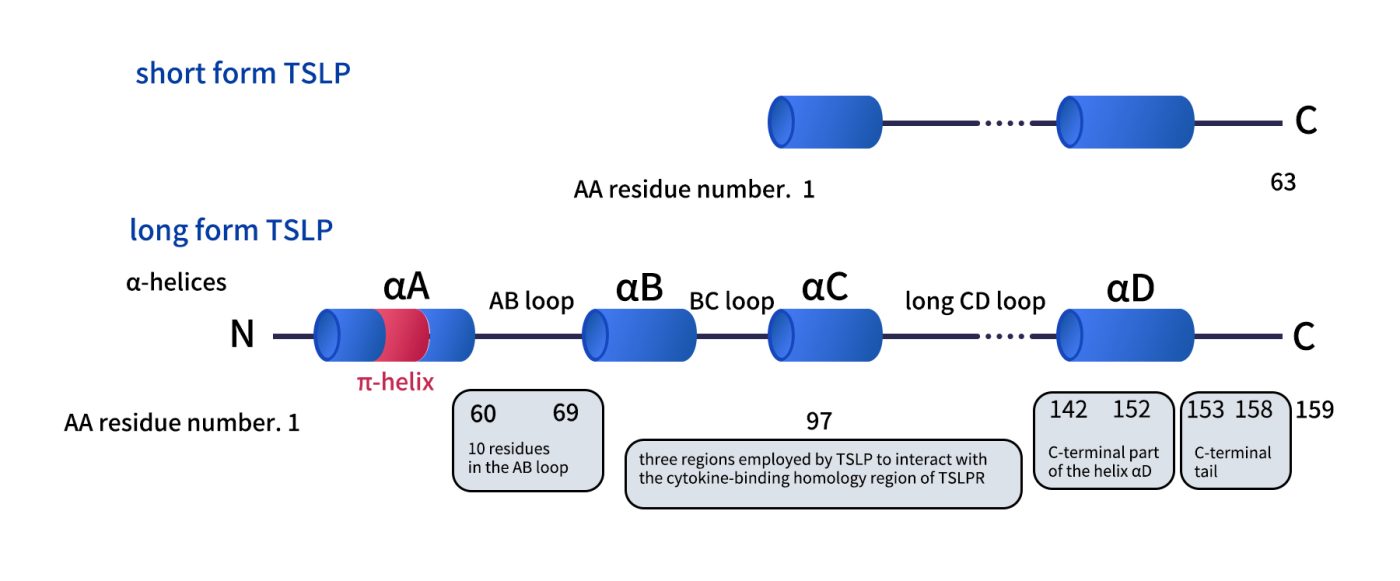
Fig 1. Comparison of thestructures of the two TSLP.[3]
- Receptor Complex
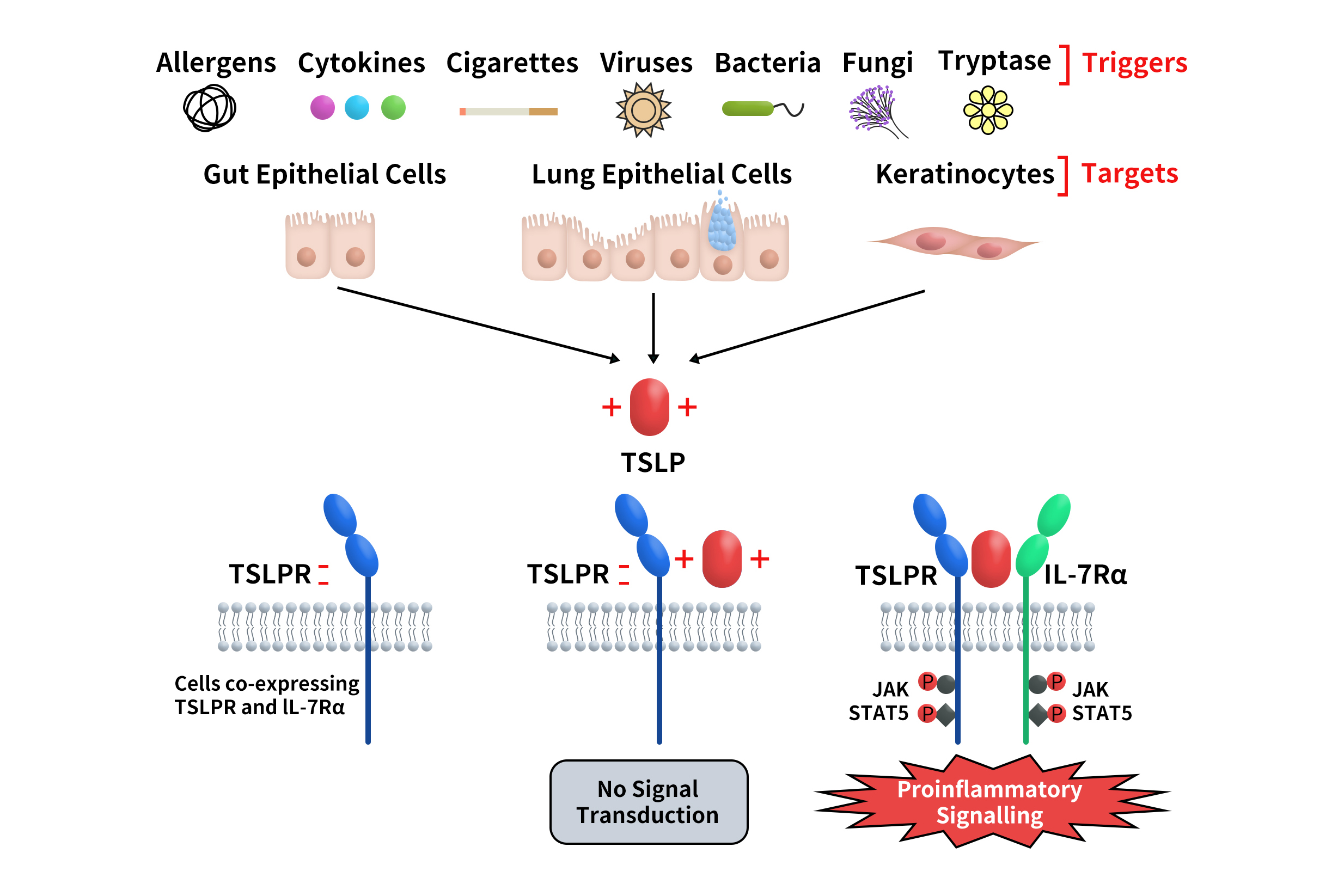
TSLP signaling is mediated through a heterodimeric receptor composed of the TSLP receptor (TSLPR, also known as CRLF2) and the IL-7 receptor alpha chain (IL-7Rα). This receptor complex is expressed on the surface of multiple immune cells, including dendritic cells (DCs), mast cells, eosinophils, B cells, and T cells. In cells co-expressing TSLPR and IL-7Rα, the formation of the ternary complex TSLPR:TSLP:IL-7Rα initiates downstream signal transduction (Fig. 2). [4]
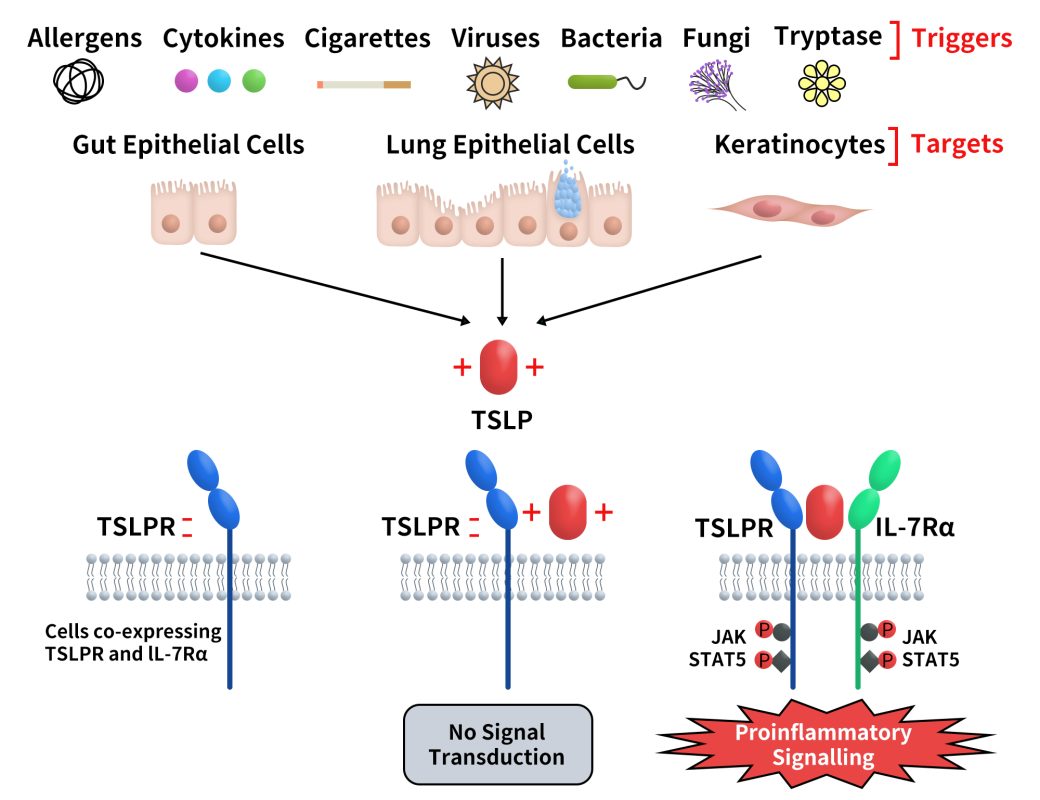
Fig 2. TSLP and its signaling complex via a cooperative stepwise mechanism on the surface of cellular targets.[4]
2. Expression Profile and Physiological Function
TSLP has been shown to be expressed in various human tissues, particularly in airway, skin, and intestinal epithelial cells. Immunologically, TSLP is considered a critical bridge between epithelial cells and the immune system. By regulating dendritic cells (DCs), T cells, and innate immune cells, TSLP induces and maintains Th2-type immune responses. [1]
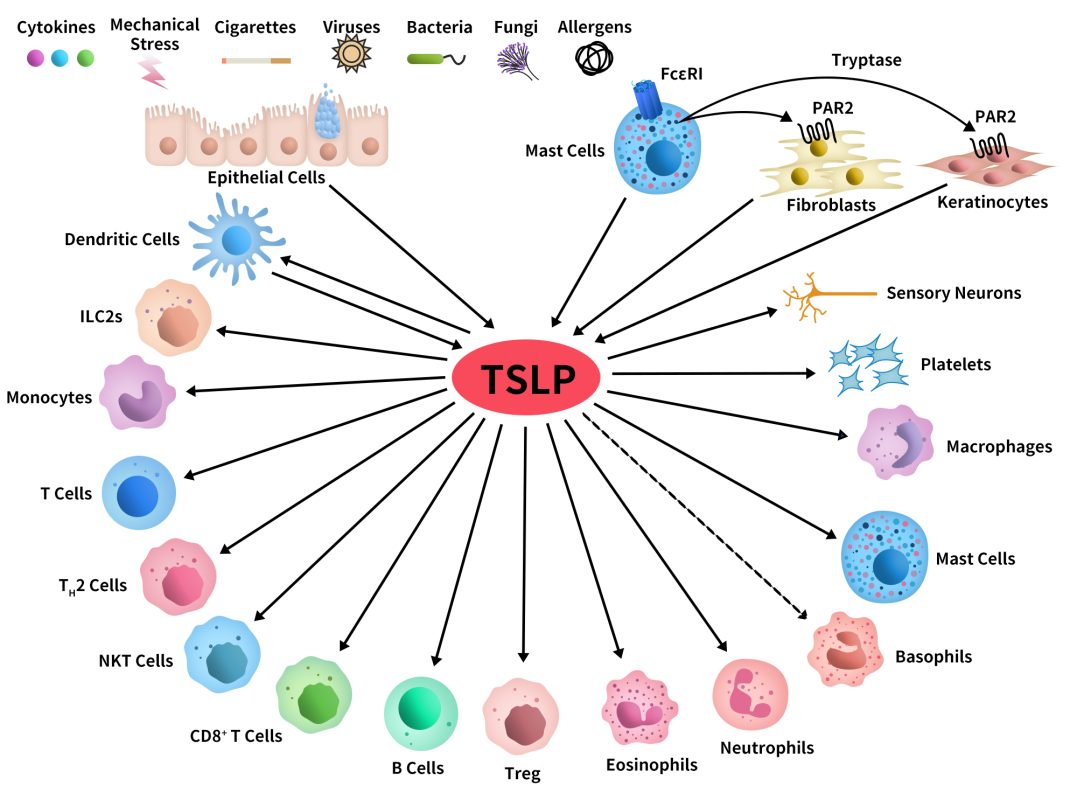
Fig 3. Schematic representation of cellular targets of TSLP.[4]
- Expression Profile
TSLP is primarily expressed in epithelial cells of barrier tissues:
Respiratory tract: Airway epithelial cells secrete TSLP upon viral infection, exposure to pollutants, or allergen stimulation.
Skin: Keratinocytes produce large amounts of TSLP in response to barrier damage.
Intestine: Intestinal epithelial cells secrete TSLP, contributing to the maintenance of intestinal homeostasis.
In addition, TSLP is expressed at low levels in certain immune cells.
- Physiological Functions
Immune barrier function: As an “epithelial cytokine,” TSLP activates DCs, bridging external stimuli with adaptive immune responses.
Promotion of Th2 responses: DCs conditioned by TSLP highly express OX40L, driving CD4⁺ T cell differentiation into Th2 cells.
Regulation of innate immunity: TSLP directly acts on mast cells, eosinophils, and basophils to enhance allergic responses.
Maintenance of immune homeostasis: Low levels of TSLP or the short-form isoform may be involved in immune tolerance and tissue repair.
3. Signal Transduction Pathways
Upon receptor activation, TSLP exerts its effects through multiple signaling cascades, including JAK-STAT, PI3K-AKT, and NF-κB pathways:
JAK-STAT pathway: TSLPR and IL-7Rα activate JAK1/JAK2, leading to phosphorylation of STAT5 and transcription of inflammatory cytokines.
PI3K-AKT pathway: Promotes survival and proliferation of immune cells.
NF-κB pathway: Induces the expression of chemokines such as CCL17 and CCL22, facilitating Th2 cell recruitment.
The synergistic action of these pathways ultimately enhances Th2-type immune responses and is closely associated with the pathogenesis of asthma and allergic diseases. [3]
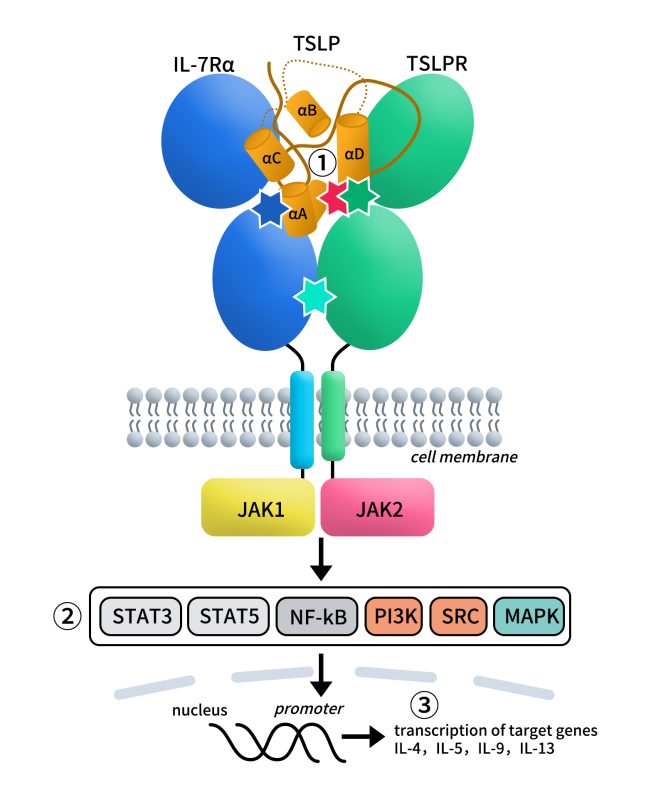
Fig 4. Graphic representation of the pro-inflammatory TSLP mediated complex and its intracellular signalling pathways.[3]
4. TSLP-Targeted Therapeutics
Currently, drug development targeting TSLP is primarily focused on monoclonal antibodies, but is gradually expanding into novel formats such as inhaled formulations, bispecific antibodies, and fusion proteins.
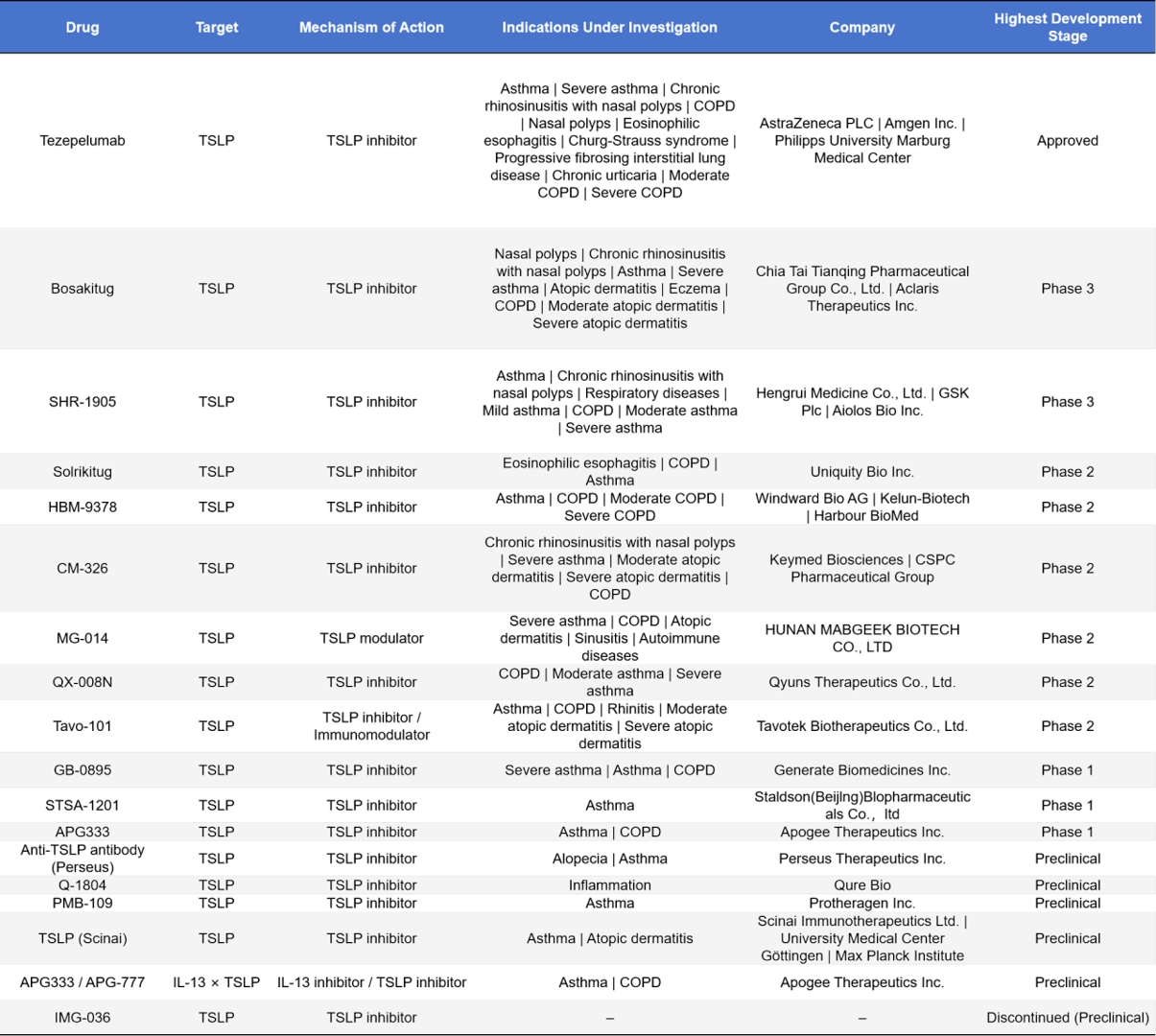
- Monoclonal Antibody Drugs
In the field of TSLP-targeted therapeutics, Tezepelumab has already been approved for the treatment of multiple allergic diseases, including asthma, nasal polyps, and eosinophilic esophagitis. Representative Phase III candidates include Bosakitug and SHR-1905, which mainly target asthma, COPD, and atopic dermatitis. The Phase II clinical pipeline is relatively diverse, with agents such as Solrikitug, HBM-9378, CM-326, MG-014, QX-008N, and Tavo-101, covering indications in respiratory and dermatological diseases. Phase I drugs, represented by GB-0895, STSA-1201, and APG333, are still under evaluation for safety and efficacy.
At the same time, multiple preclinical projects are advancing, with exploratory indications expanding to inflammation, autoimmune disorders, and even alopecia. This demonstrates the broad therapeutic potential of TSLP inhibitors in allergic and immune-related diseases.
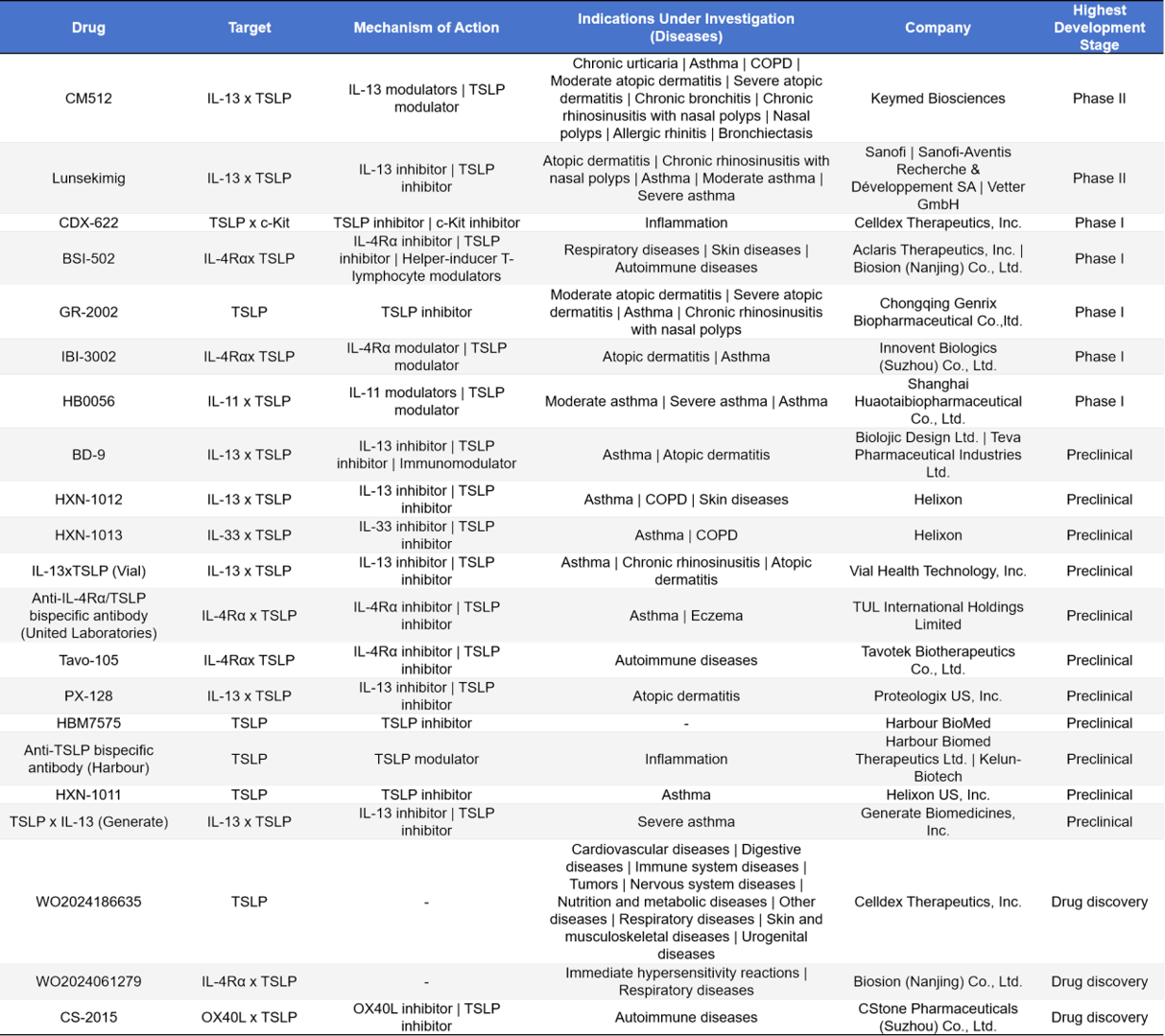
- Bispecific Antibody Drugs
In TSLP-targeted drug development, bispecific antibodies have attracted significant attention. CM512 (Keymed Biosciences ) and Lunsekimig (Sanofi) have entered Phase II clinical trials, primarily targeting asthma and atopic dermatitis. The Phase I pipeline includes diverse designs such as TSLP × c-Kit, IL-4Rα × TSLP, and IL-11 × TSLP, covering indications in respiratory, inflammatory, and autoimmune diseases. At the preclinical stage, development is even more active, with multiple companies advancing bispecific combinations of TSLP with IL-13, IL-33, IL-4Rα, and others. Overall, the TSLP bispecific antibody pipeline demonstrates diverse design strategies and broad therapeutic potential.
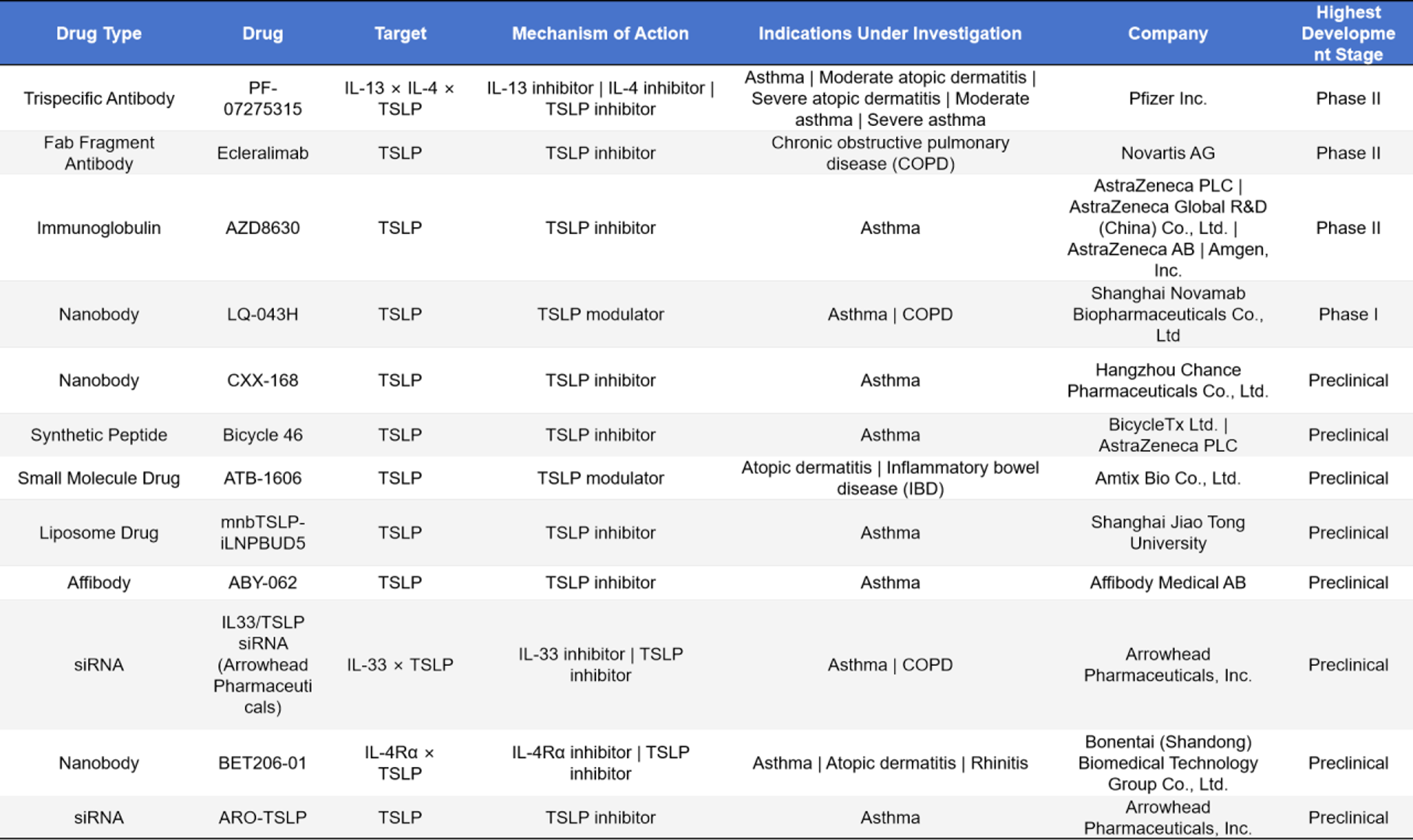
- Other Types of Drugs
In addition to monoclonal and bispecific antibodies, a variety of innovative drug modalities have emerged in the TSLP-targeted drug pipeline. Trispecific antibodies such as PF-07275315 (IL-13 × IL-4 × TSLP, Pfizer) are in Phase II clinical trials for asthma and atopic dermatitis; Fab fragment antibodies, represented by Ecleralimab (Novartis), are also in Phase II for chronic obstructive pulmonary disease (COPD); immunoglobulins such as AZD8630 (AstraZeneca/Amgen) are in Phase II for asthma. Nanobody programs include LQ-043H (Phase I, asthma/COPD) and CXX-168 (preclinical, asthma). Synthetic peptides such as Bicycle 46 (preclinical) and small-molecule modulators such as ATB-1606 (preclinical) target asthma, atopic dermatitis, and inflammatory bowel disease. Other innovative modalities include liposomal drugs (mnbTSLP-iLNPBUD5), affibody molecules (ABY-062), and siRNA drugs (IL33/TSLP siRNA and ARO-TSLP), all of which are in preclinical stages and cover indications such as asthma, COPD, and rhinitis. Overall, the TSLP-targeted drug pipeline shows broad prospects in terms of molecular diversity and indication expansion.
TSLP is a key epithelial cytokine that acts as an “upstream driver” in asthma, atopic dermatitis, and other allergic diseases. With the successful launch of Tezepelumab, TSLP has been validated as a viable therapeutic target. In the future, as research on its molecular mechanisms and functional spectrum deepens, TSLP is expected to demonstrate therapeutic potential in a wider range of diseases.
5. DIMA TSLP Target-Related Products
DIMA currently offers recombinant proteins of TSLP and its receptors (human/mouse origin), monoclonal antibodies and reference antibodies (including biotin/PE-labeled antibodies). We also provide comprehensive services, including protein/antibody customization, antibody humanization, antibody affinity maturation, and stable cell line development. In addition, we have established a B cell seed library for the TSLP target, which enables rapid screening of lead antibody molecules within 20 days according to customer requirements.For more details, please call email us at info@dimabio.com / orders@dimabio.com.
- TSLP Target-Related Proteins & Antibodies Products
- TSLP Target Lead Antibody Development Progress

References
1. Poto, R., et al., TSLP: contrasting roles in cancer. Front Immunol, 2025. 16: p. 1627235.
2. Parnes, J.R., et al., Targeting TSLP in Asthma. J Asthma Allergy, 2022. 15: p. 749-765.
3. Smolinska, S., et al., Thymic Stromal Lymphopoietin (TSLP), Its Isoforms and the Interplay with the Epithelium in Allergy and Asthma. Int J Mol Sci, 2023. 24(16).
4. Varricchi, G., et al., Thymic Stromal Lymphopoietin Isoforms, Inflammatory Disorders, and Cancer. Front Immunol, 2018. 9: p. 1595.