Over the past year, Cadherin-3 (CDH3, P-cadherin), traditionally regarded as a classical cell-cell adhesion molecule, has increasingly attracted attention in oncology drug development and international scientific conferences. Early clinical data from CDH3-targeted antibody drug conjugates (ADCs) in solid tumors, particularly non-small cell lung cancer (NSCLC), have demonstrated promising objective response signals, drawing significant interest from both academia and the biopharmaceutical industry. In parallel, bispecific antibodies and T cell redirection strategies targeting CDH3 have entered early clinical or translational exploration. Collectively, these advances suggest that CDH3 is transitioning from a tumor-associated marker to a therapeutically actionable cancer target with translational potential.
1. What Is CDH3/P-cadherin?
The CDH3 gene encodes P-cadherin, also known as placental cadherin, a member of the classical calcium-dependent cadherin family. Along with E-cadherin (CDH1) and N-cadherin (CDH2), CDH3 is a homologous classical cadherin and was the third cadherin identified and characterized in the murine visceral endoderm cell line PSA5-E. Classical cadherins mediate calcium-dependent homophilic cell–cell adhesion, playing essential roles in tissue architecture, epithelial integrity, and cell fate determination. The CDH3 gene is located on chromosome 16q22.1 and shares approximately 66% sequence homology with CDH1.
Mature P-cadherin is a 118kDa glycoprotein with a structural organization characteristic of classical cadherins. It consists of five extracellular cadherin repeats (EC1-EC5), a single transmembrane domain, and a cytoplasmic tail that mediates intracellular signaling and cytoskeletal linkage. P-cadherin primarily supports homophilic interactions between identical cadherin molecules.
The cytoplasmic domain contains two functionally critical regions: the juxtamembrane domain (JMD) and the catenin-binding domain (CBD). The JMD plays a key role in regulating cell migration, whereas the CBD is essential for cadherin function. Known interacting partners include α-, β-, γ-, and p120-catenins. β-catenin and γ-catenin are signaling molecules involved in tissue patterning, whose activity is regulated by CBD integrity, tyrosine phosphorylation, and transcriptional control. p120-catenin directly binds the JMD, modulating cadherin trafficking, stability, adhesive strength, and cell motility in a tyrosine kinase–dependent manner. α-catenin links the cadherin–catenin complex to the actin cytoskeleton and participates in signal transduction, providing the structural basis for CDH3 functioning as both a structural adhesion molecule and a signaling regulator [1].
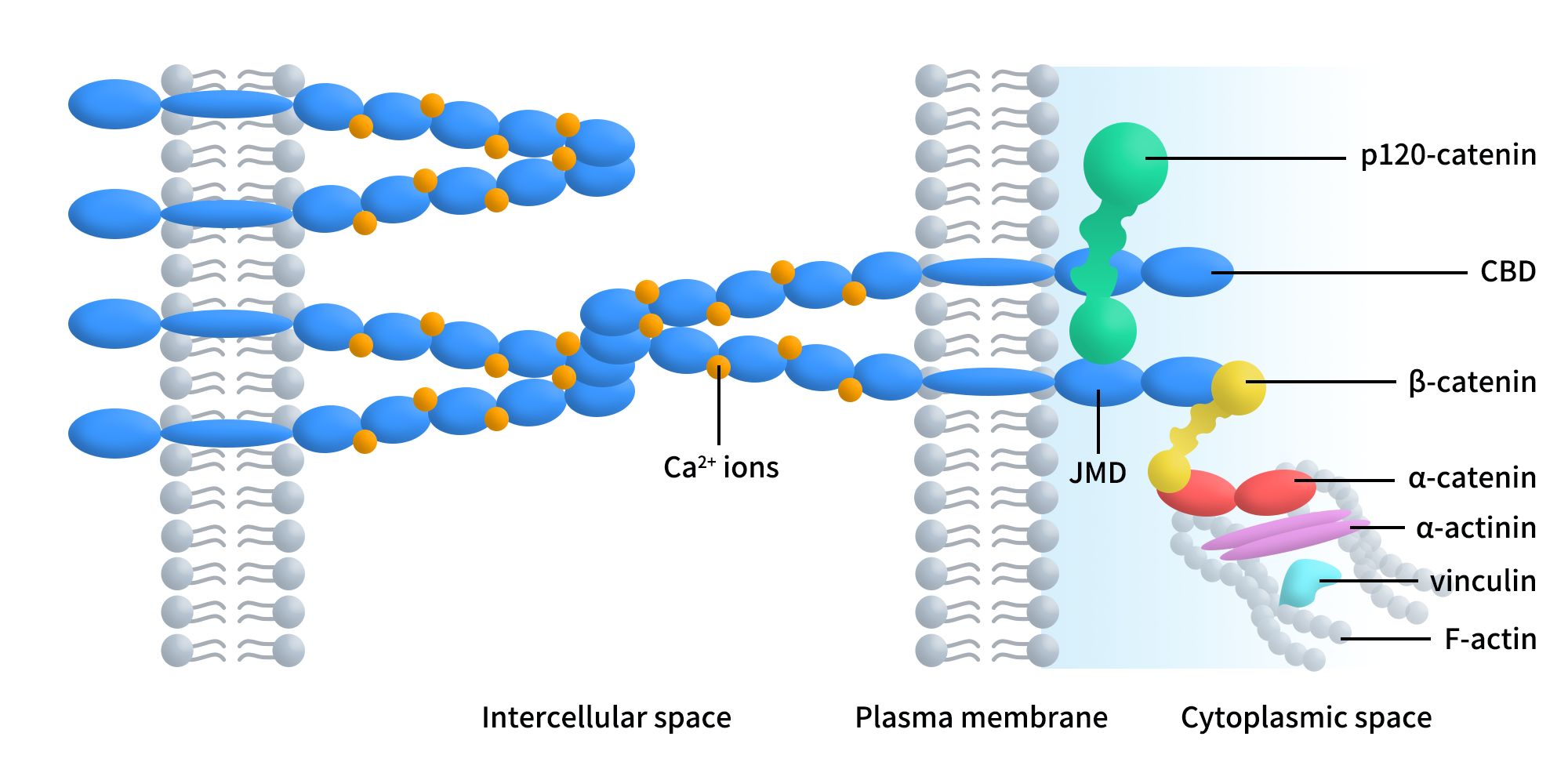
Figure 1. Schematic representation of the classical cadherin-catenin complex.[1]
Unlike CDH1, which is broadly expressed across epithelial tissues, CDH3 expression in normal adult tissues is relatively restricted, primarily observed in placental trophoblasts, subsets of basal epithelial cells, and specific developmental stages. This limited physiological expression pattern provides a theoretical safety window for tumor-targeted therapy. Under normal conditions, CDH3 contributes to cell–cell adhesion, epithelial polarity maintenance, and tissue organization, particularly during embryonic development and differentiation.
2. CDH3/P-cadherin Signaling in Tumor Cells
In cancer, CDH3 does not merely function as a passive adhesion molecule. Instead, through aberrant expression and spatial redistribution, it actively contributes to tumor invasion, migration, and malignant progression. Unlike its stabilizing role in normal epithelia, tumor-associated CDH3 functions as a signal amplification and integration hub, converting changes in adhesion status into pro-tumorigenic intracellular signaling.
A central signaling axis involves the interaction of P-cadherin with β-catenin and p120-catenin, which normally stabilize adherens junctions and link cadherins to the cytoskeleton. In malignant contexts, P-cadherin can competitively interfere with the tumor-suppressive function of E-cadherin, disrupting E-cadherin/β-catenin and E-cadherin/p120-catenin complexes. This leads to reduced cell–cell adhesion and enhanced migratory and invasive capacity.
- In basal-like breast cancer, P-cadherin interacts with α6β4 integrin, enhancing adhesion to basement membrane components such as laminin and activating FAK, Src, and AKT signaling, thereby promoting cell motility, invasiveness, and survival.
- In ovarian cancer, P-cadherin cooperates with β1 integrin and insulin-like growth factor-1 receptor (IGF-1R). P-cadherin enhances IGF-1R activation and modulates p120-catenin phosphorylation and cytoplasmic localization, facilitating early peritoneal adhesion of metastatic cells—an essential step in ovarian cancer dissemination.Moreover, P-cadherin overexpression increases the secretion and activation of MMP1 and MMP2, which degrade the extracellular matrix and cleave the extracellular domain of P-cadherin into soluble fragments with pro-invasive activity, forming a positive feedback loop that amplifies malignancy.
- In colorectal cancer models, knockdown of P-cadherin reduces β-catenin levels and downstream targets such as c-Myc and survivin, resulting in decreased invasiveness and reduced liver metastasis. P-cadherin also activates small GTPases such as Rac1 and Cdc42, influencing cytoskeletal dynamics and cell motility, and can potentiate ligand-dependent signaling of EGFR and IGF-1R, further reinforcing oncogenic signaling pathways.
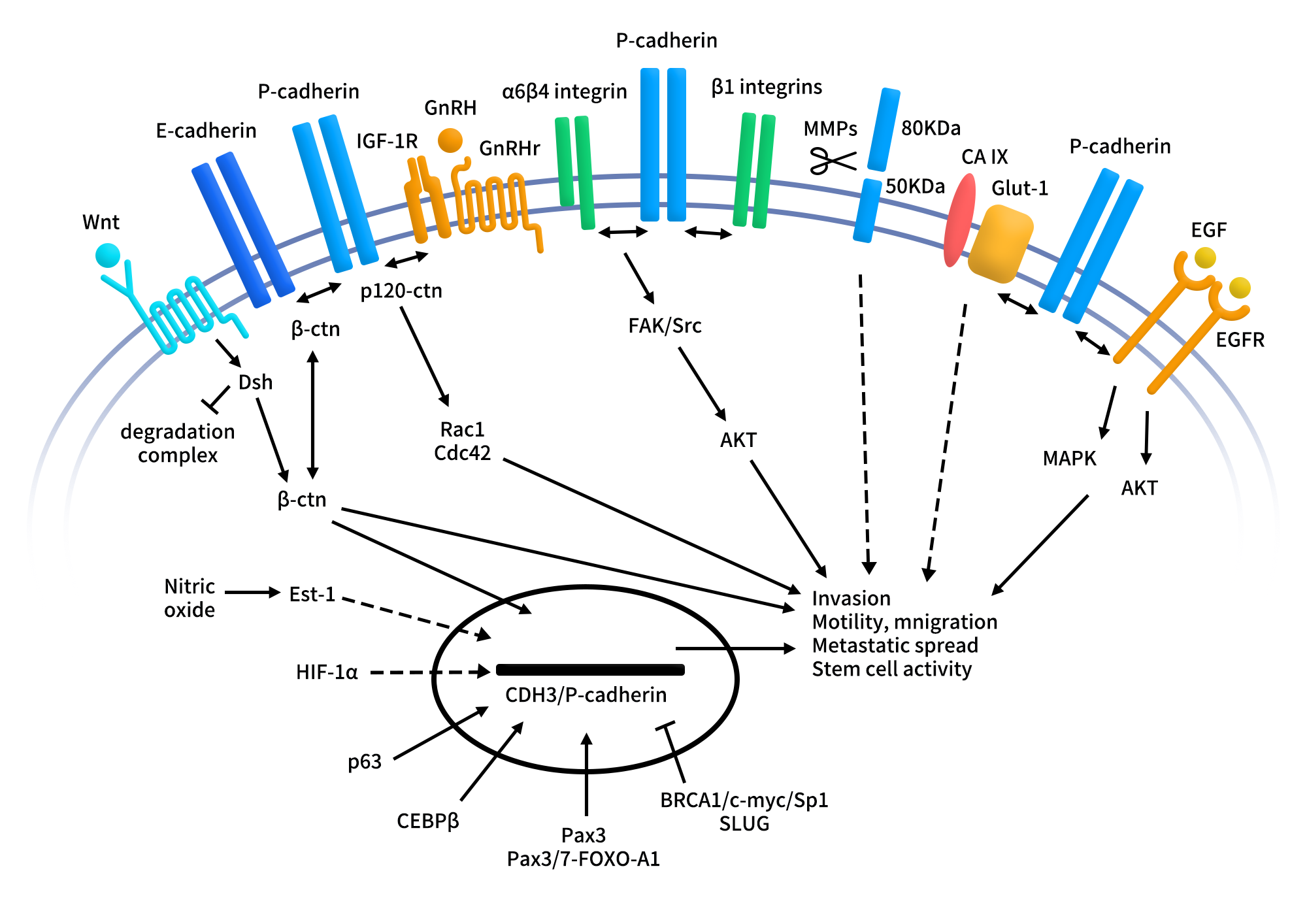
Figure 2. P-cadherin signaling pathways in the malignant setting.[2]
Collectively, the P-cadherin signaling network integrates integrins, receptor tyrosine kinases, small GTPases, and other cadherin family members, driving invasion, metastasis, and stem-like tumor phenotypes and offering multiple intervention points for therapeutic targeting.
3. Targeting CDH3/P-cadherin: Translational Value and Therapeutic Strategy
Accumulating evidence indicates that CDH3 in tumor cells functions as a signal amplifier rather than a simple adhesion molecule, cross-talking with integrins, Src/FAK/AKT pathways, small GTPases, and membrane receptors to enhance invasion and metastasis-associated signaling [2]. This functional shift is closely linked to its aberrant expression in multiple malignancies and underpins its therapeutic relevance.
- Pathologically, CDH3 is frequently upregulated in basal-like breast cancer, ovarian cancer, lung cancer, and subsets of gastrointestinal tumors, where its expression correlates with higher tumor grade, increased invasiveness, and poor prognosis [3]. Notably, CDH3 expression is often spatially enriched at invasive tumor fronts or within EMT-like and collectively migrating cell populations, suggesting that its upregulation reflects adaptive selection for enhanced migratory plasticity under microenvironmental pressure.
- From a drug development perspective, CDH3 possesses favorable targetability features. Its stable localization on the tumor cell surface and accessible extracellular cadherin domains make it well suited for antibody-based targeting and payload delivery, while its limited expression in normal adult tissues supports a potential therapeutic window [4]. However, mechanistic studies also indicate that CDH3 is not a classical oncogenic driver but rather a functional facilitator within invasive signaling networks. As such, directly inhibiting its signaling may be insufficient to suppress tumor growth, whereas exploiting CDH3 as a tumor-selective targeting moiety is particularly attractive.
Consequently, current CDH3-targeted strategies emphasize ADCs, bispecific antibodies, and combination approaches with targeted or immune therapies, leveraging CDH3’s tumor-associated surface expression for selective therapeutic delivery. Patient stratification based on CDH3 expression levels, spatial distribution, and invasion-associated phenotypes is increasingly recognized as a key factor for successful clinical translation.
4. Clinical Development of CDH3-Targeted Therapies
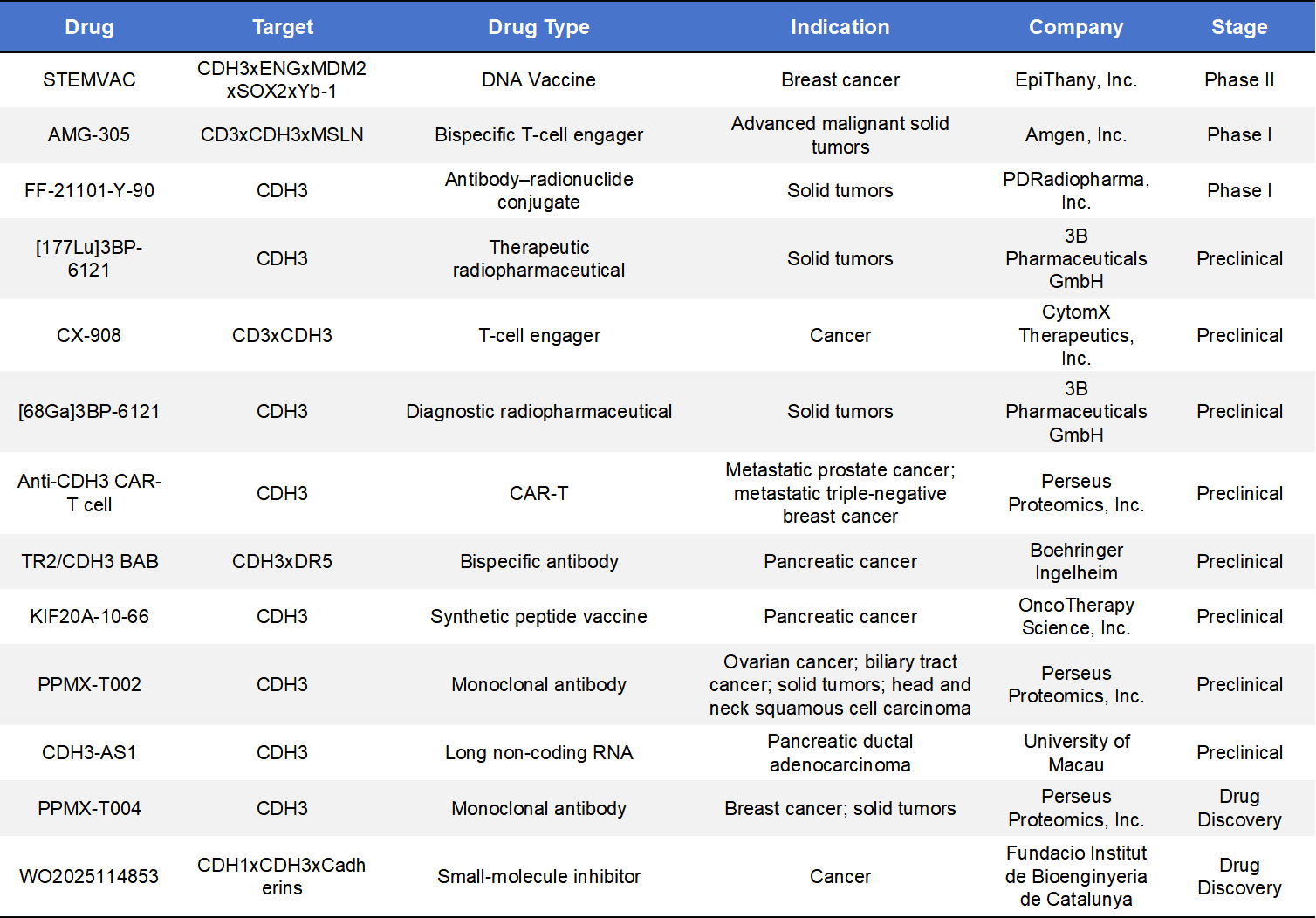
At present, CDH3-targeted therapies are steadily advancing into clinical development. According to incomplete statistics, approximately 26 CDH3-targeting agents are under development globally, including four in clinical trials and twelve in preclinical stages, with ADC-based modalities predominating among active programs.
4.1 CDH3-Targeted ADCs
As an ADC target, CDH3 is highly expressed on the surface of tumor cells, exhibits strong tumor selectivity, and demonstrates efficient internalization, enabling precise delivery of cytotoxic payloads while minimizing off-tumor toxicity. Based on these advantages, multiple biopharmaceutical companies have initiated CDH3-focused ADC development programs.
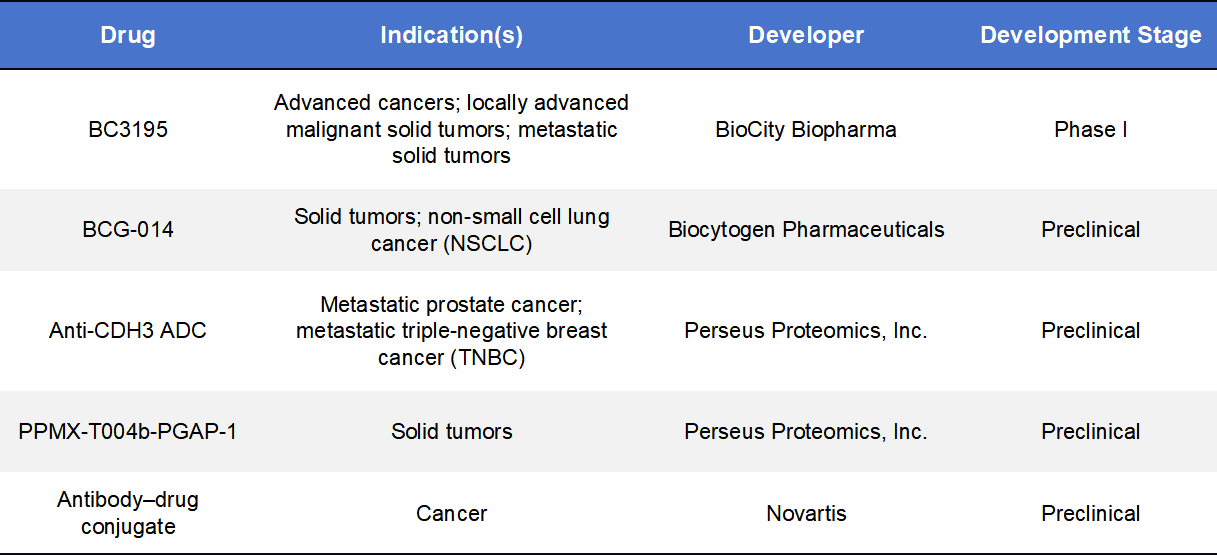
BC3195, developed by BioCity Biopharma, is the first-in-class CDH3-targeted ADC. It consists of a high-affinity anti-CDH3 monoclonal antibody conjugated to the microtubule inhibitor MMAE via a cleavable linker. Upon binding to CDH3 on tumor cells, BC3195 undergoes internalization and intracellular payload release, inducing cell-cycle arrest and apoptosis. BC3195 is currently in Phase I clinical trials in China and the United States, including dose-escalation and expansion cohorts. Early data demonstrate encouraging antitumor activity, particularly in advanced solid tumors such as NSCLC, with manageable safety profiles, validating the clinical feasibility of CDH3 as an ADC target.
BCG-014, developed by Biocytogen Pharmaceuticals, is a novel CDH3-targeted ADC candidate featuring a topoisomerase I (TOP1) inhibitor payload, distinguishing it from traditional microtubule-based ADCs. This design enables DNA damage-mediated cytotoxicity in addition to conventional mechanisms. BCG-014 is currently in the preclinical stage and has demonstrated robust antitumor efficacy in multiple patient-derived xenograft (PDX) models. Ongoing studies are evaluating its pharmacokinetics, efficacy, and safety to support clinical advancement.
Additional CDH3-targeted ADC programs are being pursued by Perseus Proteomics and Novartis, although these remain at preclinical stages.
4.2 CDH3 Bispecific Antibodies and Other Modalities
Beyond ADCs, monoclonal antibodies and bispecific antibodies (BsAbs) represent important alternative CDH3-targeting strategies. One example is PPMX-T002, a CDH3-directed monoclonal antibody developed by Perseus Proteomics, currently in preclinical evaluation for its antitumor potential in biliary tract cancer, ovarian cancer, and other solid tumors.
Multiple CDH3xCD3 bispecific antibody programs are also under development. For instance, Creative Biolabs has advanced a CDH3xCD3 DART-based construct designed to physically link T cells with CDH3-expressing tumor cells, thereby triggering T-cell-mediated cytotoxicity. Preclinical immuno-oncology studies support the mechanistic rationale of this approach. Additionally, TRAILR2/CDH3 bispecific antibody concepts have been reported, promoting tumor cell apoptosis through dual receptor engagement and demonstrating selective antitumor activity in pancreatic and other solid tumor models.
Beyond antibody-based therapeutics, academic research has explored correlations between CDH3 expression and sensitivity to small-molecule therapies, suggesting that indirect strategies, such as combination with EGFR inhibitors, may enhance therapeutic benefit. However, no direct small-molecule CDH3 inhibitors have yet entered clinical trials. Compared with ADC platforms, these non-ADC modalities remain at earlier stages of development but offer broader possibilities for future combination and immune-modulating therapies.
5.DIMA BIOTECH’s CDH3 Research Tools for Antibody Discovery & Functional Validation
DIMA BIOTECH offers a comprehensive CDH3 research toolkit to accelerate antibody discovery and functional studies. Our portfolio includes multi-species and multi-epitope recombinant CDH3 proteins, live-cell flow cytometry-validated antibodies, and reference antibodies, enabling robust epitope mapping, antibody screening, and functional validation across diverse tumor models.
- CDH3-products
|
SKU |
Product_type |
Product name |
|
PME100802 |
ECD Proteins |
|
|
PME101596 |
||
|
PME-M100118 |
||
|
PME-C100077 |
||
|
PME101613 |
||
|
PME101614 |
||
|
PME101615 |
||
|
PME101616 |
||
|
DMC101041 |
Monoclonal antibodies |
|
|
DMC101041B |
||
|
DMC101041P |
- CDH3 lead antibody molecule research progress

References
- Paredes J, Correia AL, Ribeiro AS, Albergaria A, Milanezi F, Schmitt FC. P-cadherin expression in breast cancer: a review. Breast Cancer Res. 2007;9(5):214.
- Vieira AF, Paredes J. P-cadherin and the journey to cancer metastasis. Mol Cancer. 2015 Oct 6;14:178.
- Albergaria A, et al. P-cadherin role in normal breast development and cancer. International Journal of Developmental Biology, 2011.
- Ribeiro AS, et al. P-cadherin signals through the laminin receptor α6β4 integrin to induce stem cell and invasive properties. Molecular Oncology, 2014.
