In today’s drug discovery landscape, membrane proteins remain one of the most important target classes. G protein–coupled receptors (GPCRs), ion channels, and membrane transporters play central roles in signal transduction, cell–cell communication, and disease pathogenesis. It is estimated that more than 60% of approved small-molecule drugs act on membrane protein targets.However, in the field of antibody therapeutics, membrane proteins have long been regarded as difficult or high-risk targets. Their high hydrophobicity, structural complexity, and strong dependence on lipid environments make them particularly challenging to handle. Once removed from the native membrane, membrane proteins often undergo conformational collapse, epitope loss, or functional inactivation, which directly compromises two critical steps in antibody discovery: immunization and screening.
To overcome these limitations, researchers have developed Nanodisc technology, originally designed to address membrane protein expression and stabilization challenges. Over the past decade, Nanodiscs have gradually emerged as a key enabling platform for membrane protein antibody development, with multiple studies demonstrating robust and reproducible value across different targets and modalities.
1. Overview of Nanodisc Technology
Nanodiscs are biomimetic lipid nanoparticle systems designed to stabilize and present membrane proteins in aqueous environments. Their defining feature is the ability to construct a size-controlled, composition-defined lipid bilayer microenvironment using membrane scaffold proteins or other stabilizing agents. This architecture enables membrane proteins to remain embedded in a lipid bilayer, thereby preserving their native conformation and functional state. As the technology has evolved, Nanodisc systems have expanded beyond early membrane scaffold protein (MSP)–dependent formats to include multiple stabilization strategies tailored to different classes of membrane proteins. For more details on the classification and characteristics of Nanodiscs, please read >>
This combination of functional consistency and technical flexibility has positioned Nanodiscs as a widely adopted, platform-level solution in membrane protein research and antibody discovery, rather than a single, narrow technical approach. Compared with traditional detergent micelles or membrane fragments, the core advantage of Nanodiscs lies in their ability to maintain membrane proteins in a native-like lipid context while remaining experimentally tractable. As a result, Nanodiscs are increasingly used not only in structural biology and drug screening, but also in antibody immunization and screening workflows. But do Nanodiscs truly make a decisive difference in antibody development? A compelling GPCR nanobody case study provides a clear answer.
2. How APJ-Nanodiscs Enabled the Discovery of the Single-Domain Antibody JN241-9
In a study published in Science Advances, researchers focused on the Apelin receptor (APJ), a class A GPCR with significant therapeutic potential in cardiovascular and metabolic diseases. Despite its importance, antibody development against APJ had long been hindered by conformational instability and poor preservation of functional epitopes. In this work, APJ-Nanodiscs were used consistently across both immunization and phage display screening, forming the foundation of the entire antibody discovery strategy.
2.1 APJ-Nanodiscs as Immunogens
The researchers first reconstituted a thermostabilized human APJ full-length membrane protein into Nanodiscs, generating a well-defined APJ-Nanodisc complex. This complex was then used to immunize alpacas for the construction of a single-domain antibody (VHH) library.
This immunization strategy offered several key advantages:
- The immune system was exposed to a full-length GPCR embedded in a lipid environment
- Multi-pass transmembrane regions and conformation-dependent epitopes were preserved
- Antibody responses biased toward native structural and functional regions, rather than truncated or misfolded fragments
In essence, Nanodiscs significantly increased the probability of generating functional antibodies at the immunization stage.
2.2 Using the Same APJ-Nanodiscs for Phage Display Screening
Equally important, the study did not switch antigen formats during screening. The same APJ-Nanodisc construct was reused as the antigen in phage display selection, enriching for high-affinity, conformation-sensitive antibody clones.
This immunogen-to-screening consistency ensured that:
- Antibodies generated during immunization were well matched to the screening antigen
- Selection favored antibodies recognizing the native conformation of APJ
- The risk of immunization-positive but screening-negative outcomes was dramatically reduced
Using this integrated strategy, the researchers identified multiple APJ-binding nanobodies and, through structural analysis and rational design, ultimately developed JN241-9, a candidate single-domain antibody with agonist activity. This case clearly demonstrates that Nanodiscs function not merely as passive carriers, but as core technical components spanning the entire antibody discovery workflow [1].
3. Core Advantages of Nanodiscs in Membrane Protein Antibody Development
Based on this study and broader research experience, Nanodiscs provide several critical advantages in membrane protein antibody discovery:
01 Preservation of native conformation and higher functional antibody yield
Lipid-stabilized environments help maintain the structural integrity of GPCRs, ion channels, and other membrane proteins, increasing the likelihood of generating antibodies against pharmacologically relevant epitopes.
02 Unified use in both immunization and screening
Using the same antigen format throughout the workflow minimizes false positives and functional loss caused by antigen inconsistency.
03 Particularly well suited for nanobody and VHH development
Single-domain antibodies are highly conformation-sensitive, and Nanodiscs show strong synergy with camelid immune systems.
04 Highly compatible with complex targets
For GPCRs and multi-pass membrane proteins, Nanodiscs outperform soluble extracellular fragments [2][3][4].
As Nanodisc applications mature, a practical question naturally arises: Should Nanodiscs be prioritized as immunogens in membrane protein antibody projects?
4. Nanodisc vs. Multi-Fragment Proteins: Choosing the Right Immunogen Strategy
In many antibody discovery programs, multi-fragment proteins (see more on their use in therapeutic antibody development…) remain a widely adopted and efficient traditional approach. Compared with Nanodiscs, these two immunogen formats address different scientific and project needs. Understanding their differences helps guide rational decision-making across development stages [5].
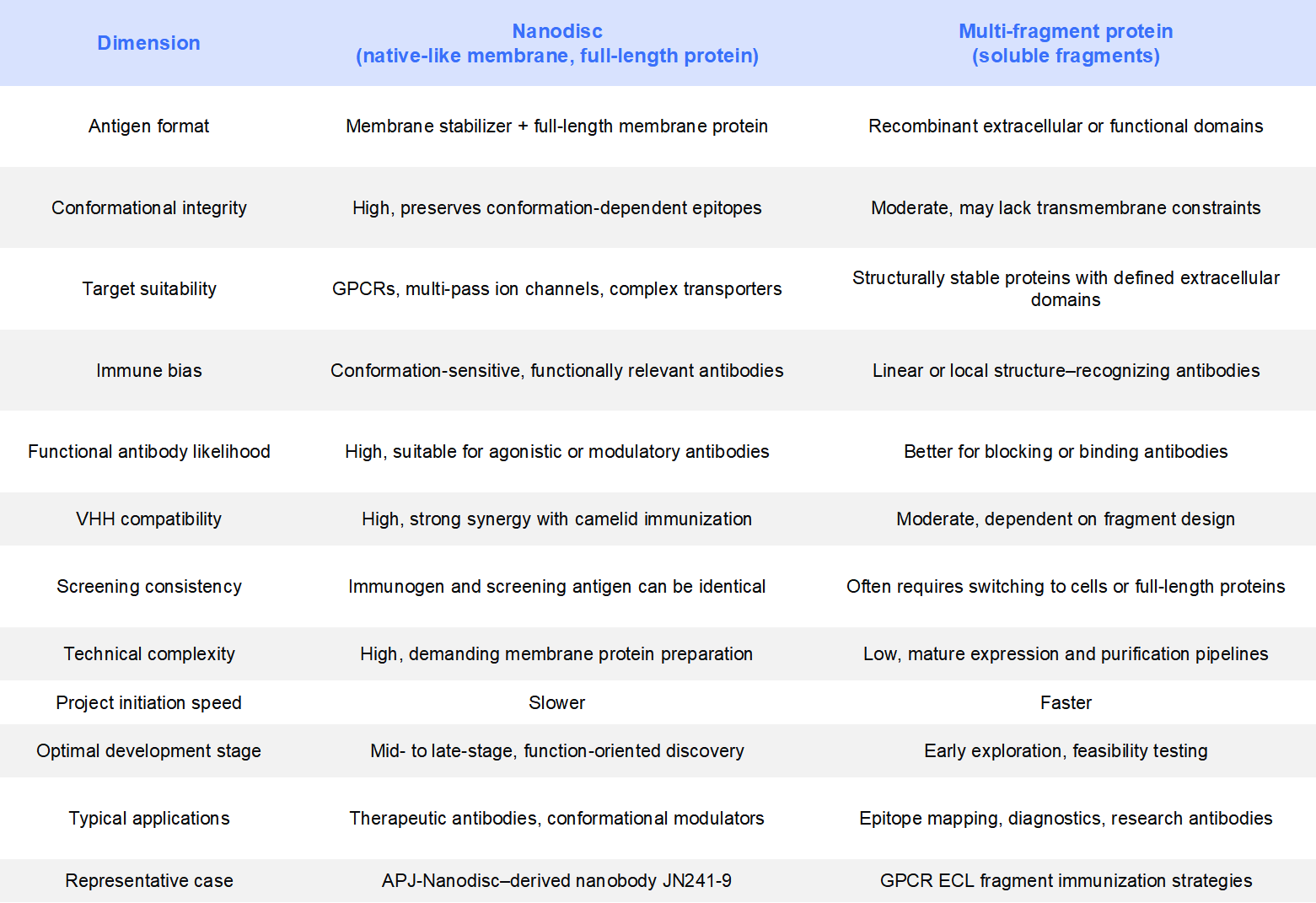
In real-world antibody development, immunogen selection should always serve the final therapeutic objective. When targets exhibit complex conformational dynamics, rely on transmembrane domain coordination, or when the goal is to generate functional or modulatory antibodies, Nanodiscs often offer a higher long-term success rate. Conversely, during early-stage exploration—where speed, feasibility assessment, or epitope mapping is prioritized—multi-fragment proteins remain an efficient and practical choice.
Reference:
- Ma Y, et al. Structure-guided discovery of a single-domain antibody agonist against the apelin receptor. Science Advances. 2020.
- Kijimoto-Ochiai S, et al. Nanodisc-based immunization strategies for membrane protein antibody discovery. Front Immunol. 2021.
- Hino T, et al. Antibody-based GPCR modulation: structural insights and therapeutic potential. Nat Struct Mol Biol. 2019.
- Manglik A, et al. Structural insights into GPCR signaling using nanodisc technology. Nature. 2015.
- Lee SC, et al. Strategies for antibody discovery against challenging membrane proteins. mAbs. 2021.