Despite the historical success of PD-1/PD-L1 inhibitors, over 60% of cancer patients remain unresponsive, highlighting the need for next-generation immunotherapy targets capable of converting “cold tumors” into “hot tumors.” Recently, STAB1 (Stabilin-1, also known as Clever-1) has emerged as a promising target due to its unique role in regulating macrophage phenotype and tumor immune evasion.
1. STAB1 / Stabilin-1 and the Scavenger Receptor Family
Stabilin-1, encoded by the STAB1 gene, is a large multifunctional type I transmembrane receptor belonging to the Class H Scavenger Receptors (SRs). Scavenger receptors are a diverse family of proteins originally identified for their ability to recognize modified low-density lipoproteins. Based on structural characteristics, SRs are divided into classes A through L. Stabilin-1, together with Stabilin-2, defines the H class, exhibiting typical “multi-ligand recognition” features, including phosphatidylserine, advanced glycation end products (AGEs), and various extracellular matrix components [1,2].
2. STAB1 / Stabilin-1 Structure
Human Stabilin-1 is a large transmembrane protein (~280–310 kDa, 2570 amino acids), with a multi-domain extracellular region responsible for complex ligand interactions [1]. Its extracellular domain contains 7 Fasciclin-like (FAS1) domains, 16 canonical EGF-like domains, and 1 C-type lectin-like Link domain. EGF-like repeats are often organized into clusters in the stabilin family. Transmembrane region anchors STAB1 to the plasma membrane. Cytoplasmic tail is short but contains functional motifs (e.g., DDSLL, acidic clusters) mediating interactions with intracellular sorting adaptors (GGA1/2/3, SNX17). Among of them, fasciclin domains facilitate protein-protein interactions, acting as binding sites for ligands such as SI-CLP and placental lactogen. EGF-like domains contain disulfide bonds stabilizing extended conformations; participate in ligand binding (e.g., SPARC), protein stability, and signaling. link domain potentially interacts with glycosylated ligands and stabilizes receptor conformation. Cytoplasmic tail regulates receptor endocytic recycling and downstream signaling.
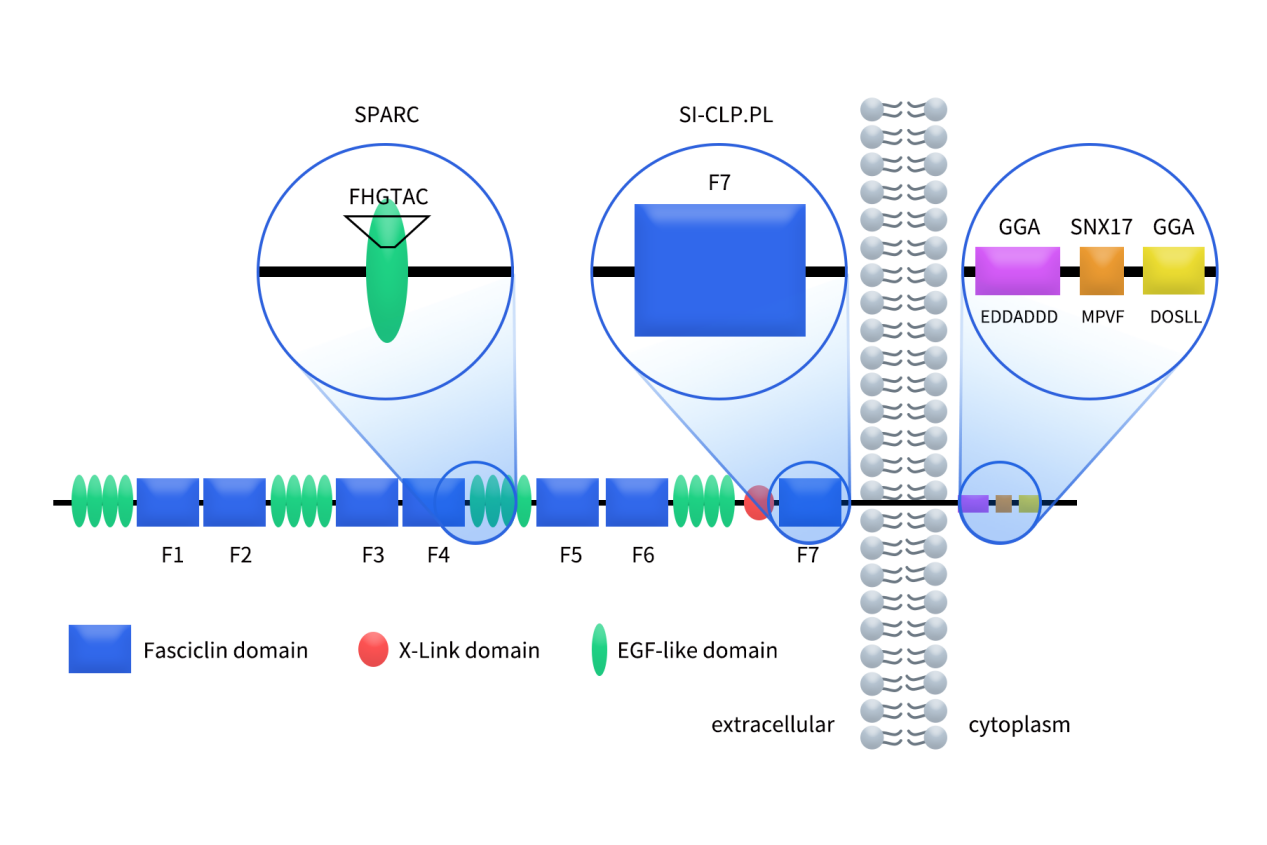
Figure 1: Human STAB1/Stabilin-1 structure.
3. STAB1/Stabilin-1 Distribution and Function
STAB1 is highly expressed in liver sinusoidal endothelial cells, spleen, lymph nodes, bone marrow, and adrenal vasculature, as well as in circulating non-classical monocytes and M2-polarized macrophages [2]. Unlike SR-AI or CD36, Stabilin-1 exhibits high intracellular recycling efficiency, roles in intracellular protein sorting (TGN-endosome trafficking), clearance of extracellular toxins, and regulation of profibrotic factors (e.g., SPARC). STAB1 is enriched in tumor-associated macrophages (TAMs) in melanoma, breast cancer, colorectal cancer, and glioblastoma, forming a key barrier to anti-tumor immunity [3]. High STAB1 expression correlates with poor patient survival, making it a critical target to restore adaptive immune activation in the TME.
4. STAB1/Stabilin-1 Mechanism: Tumor Microenvironment Immune Switch
STAB1 acts as a multifunctional scavenger receptor on TAMs, modulating immune suppression, antigen clearance, inflammation regulation, and T cell infiltration. Mechanistically, STAB1 mediates “silent clearance” of tumor-suppressive factors (e.g., SPARC), avoiding strong pro-inflammatory macrophage polarization. STAB1-high TAMs typically display low expression of pro-inflammatory markers (TNFα, IL-12), high expression of M2 markers (CD206, CD163), and suppressed anti-tumor inflammation. STAB1 inhibition reverses this phenotype toward M1 polarization, enhances CD8+ T cell infiltration, restores cytotoxic T cell activity, and affects T cell priming in lymph nodes.
Targeting STAB1 with antibodies induces increased pro-inflammatory cytokines (IFNγ, TNFα, CCL3), M2-to-M1 macrophage transition (upregulation of CD86, antigen-presenting receptors), downregulation of CD163/CD206, modulation of Treg function, and recruitment and activation of CD8+ T cells. Overall, STAB1 blockade leads to reduced tumor growth and increased tumor cell apoptosis.
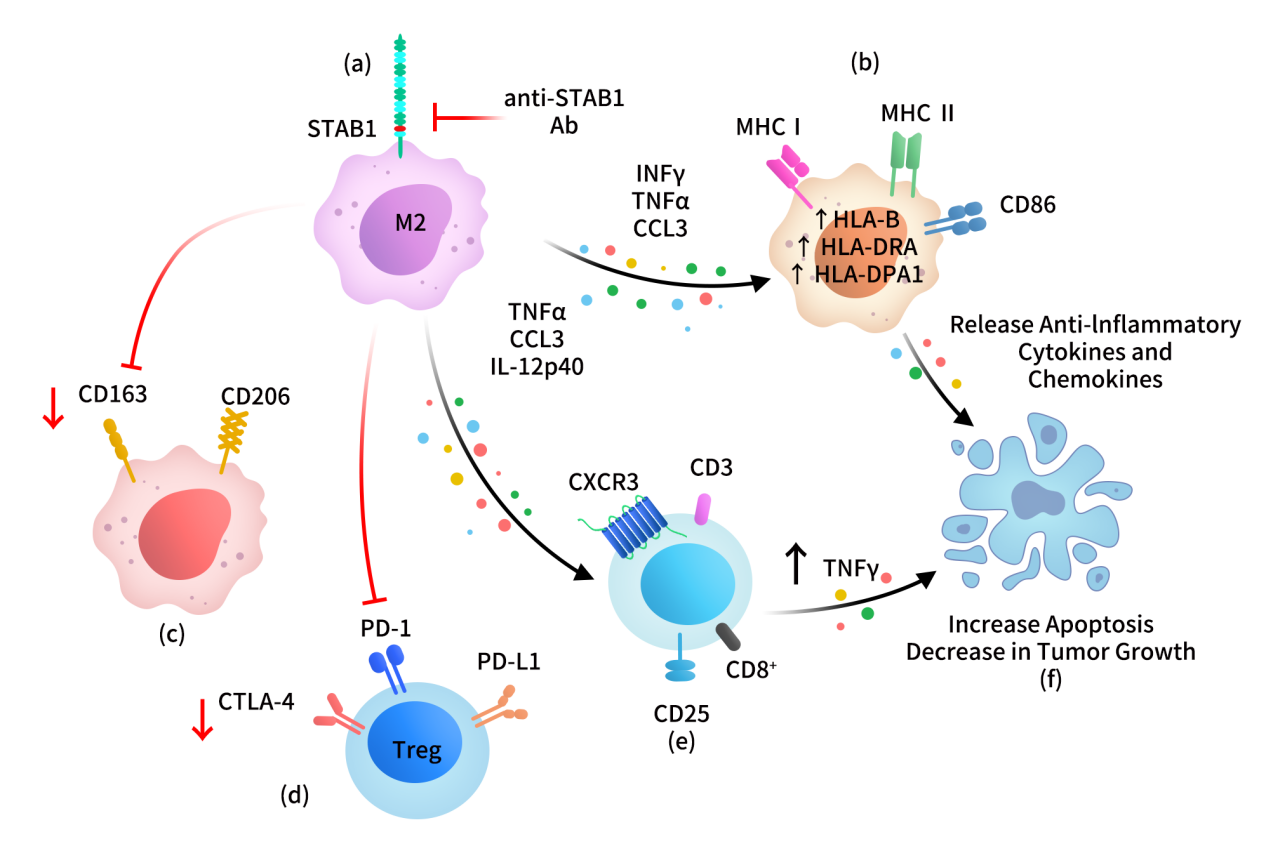
Figure 2: Targeting STAB1 in TAMs to enhance anti-tumor immunity. [4]
5. Clinical Research Progress of STAB1 Targeting
The only STAB1-targeted therapeutic in clinical development is Bexmarilimab (FP-1305), a fully human IgG4 monoclonal antibody against CLEVER-1, developed by Faron Pharmaceuticals. Its mechanism is reprogramming TAMs from M2 to M1, enhance antigen presentation and IFNγ signaling, and increase of T cell infiltration and anti-tumor immunity.
Nonclinical data confirm Bexmarilimab inhibits CLEVER-1 ligand internalization, reduces immune suppression, and enhances TNFα secretion without significant cytokine release syndrome or Fcγ receptor-mediated off-target effects [5]. Currently, it has two active clinical trials, MATINS (NCT03733990) and BEXMAB.
- MATINS (NCT03733990) is a Phase I/II study of Bexmarilimab monotherapy in advanced solid tumors (e.g., colorectal cancer, cholangiocarcinoma), and is demonstrated safety, tolerability, increased T cell infiltration, and TAM phenotype conversion in PD-1/L1 resistant patients [6].
- BEXMAB also is a Phase I/II study in hematologic malignancies. Combined with azacitidine for high-risk MDS and relapsed/refractory AML, achieved 45% ORR, particularly in hypomethylation-resistant subgroups. Combination with azacitidine/venetoclax enhanced HLA-DR expression and inhibited tumor cell viability, suggesting synergistic multi-drug potential.
STAB1 inhibitors are being explored as immune sensitizers, combined with PD-1 inhibitors, chemotherapy, or other targeted therapies to overcome myeloid cell-mediated resistance, offering new hope for immunotherapy non-responders.
6. DIMA BIOTECH’s STAB1 Research Tools
DIMA BIOTECH provides STAB1/CLEVER-1 recombinant proteins and reference antibodies for immunological assays, antibody screening, ligand binding and cell surface expression evaluation, and affinity analysis. DIMA BIOTECH is also leveraging an innovative single-B cell platform to accelerate STAB1 antibody lead discovery and welcomes collaboration and testing inquiries.
|
Product Type |
Cat.No. |
Product Name |
|
ECD Protein |
PME100719 |
|
|
PME100718 |
||
|
PME-M100036 |
||
|
PME-M100035 |
||
|
Reference Antibody |
BME100434B |
|
|
BME100434 |
References
- Murphy, A. J., et al. (2005). Stabilin-1 and -2 constitute a novel family of fasciclin-like hyaluronan receptor homologues. Biochemical and Biophysical Research Communications, 328(4), 928-933.
- PrabhuDas, M., et al. (2017). A consensus definitive classification of scavenger receptors and their roles in health and disease. Journal of Immunology, 198(11), 4175-4189.
- Palani, S., et al. (2016). Stabilin-1 is expressed in human breast cancer and supports tumor growth in mammary adenocarcinoma mouse model. Oncotarget, 7(15), 19308-19321.
- Gurung, J. L., Tamang, R. L., Madduri, L., et al. (2025). Stabilin-1 in Tumor-Associated Macrophages: A Potential Therapeutic Target in Cancer Immunotherapy. Biology, 14(9), 1198.
- Hollmén M, Maksimow M, Rannikko JH, et al. Nonclinical Characterization of Bexmarilimab, a Clever-1-Targeting Antibody for Supporting Immune Defense Against Cancers. Mol Cancer Ther. 2022 Jul 5;21(7):1207-1218.
- Virtanen, S., et al. (2024). Bexmarilimab, a macrophage-reprogramming antibody, in patients with advanced solid tumors: The MATINS phase I/II trial. Nature Communications/Journal updates.
