In recent years, the role of chemokine receptors in immune regulation, tumor development, and tissue repair has attracted increasing attention. C-X-C chemokine receptor type 7 (CXCR7), also known as ACKR3 (Atypical Chemokine Receptor 3), serves as an atypical receptor for CXCL12/CXCL11. Owing to its unique signaling mechanism and functional roles in various diseases, CXCR7 has emerged as a promising new target for drug development.
1. CXCR7 Structure and Expression
1.1 Structure
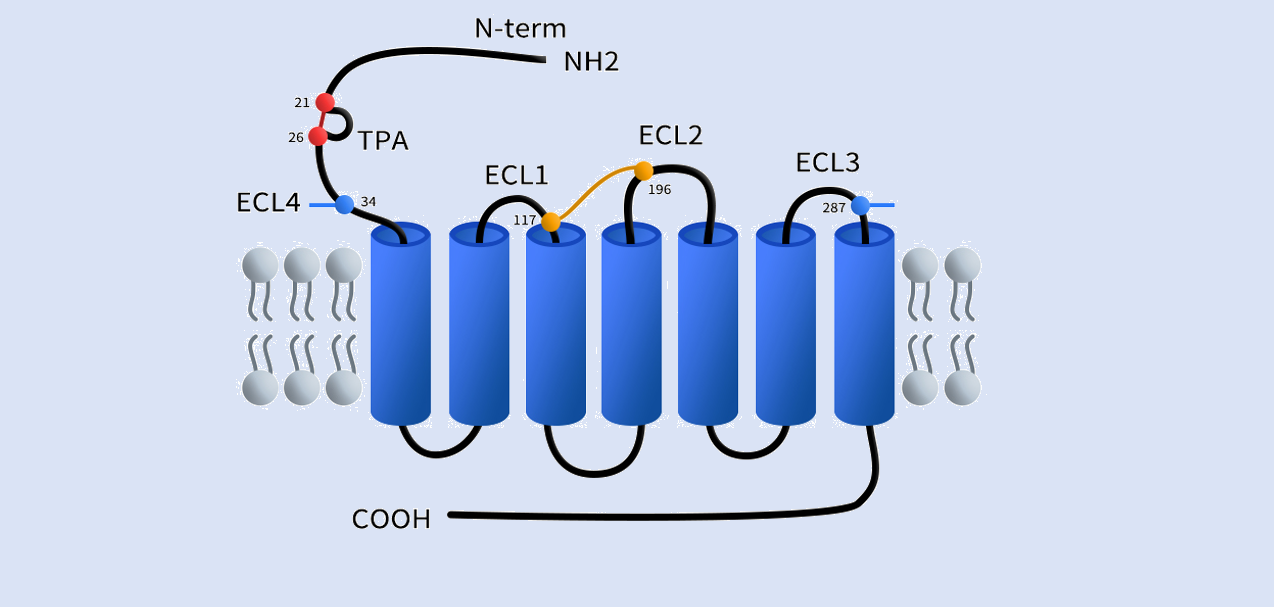
CXCR7 belongs to the atypical GPCR family (ACKRs) and displays several structural features that are distinct from typical GPCRs, particularly in its extracellular regions. First, the N-terminus of CXCR7 is unique, with cysteine residues at positions 21 and 26 forming a characteristic disulfide bond that creates a stable N-terminal loop. This loop structure is believed to be closely related to ligand recognition and receptor stability. In addition, CXCR7 possesses another important disulfide bond between the seventh transmembrane helix (TM7) and the extracellular loop above it (commonly referred to as ECL4), a feature rarely seen in most GPCRs. This structural element confers CXCR7 with distinctive conformational stability and specificity, facilitating its high-affinity binding to chemokines such as CXCL12 and CXCL11. The presence of these disulfide bonds not only enhances the structural stability of CXCR7’s extracellular domain but also plays a crucial role in its atypical functions in modulating chemokine activity, regulating cell migration, and controlling GPCR signal transduction. [1]
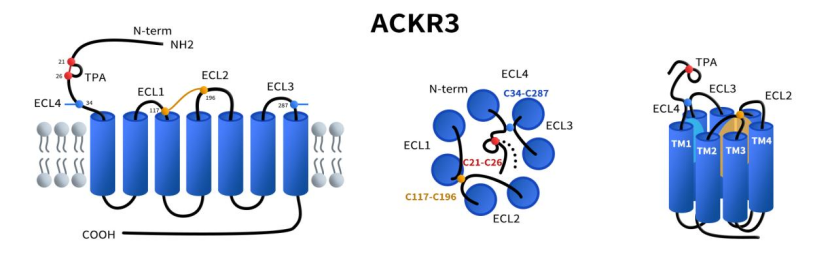
Fig 1. The struture of chemokine receptor CXCR7(ACKR3).[1]
1.2 Expression
CXCR7 is expressed in human B cells, dendritic cells, and tumor-associated endothelial cells, and is involved in regulating CXCR4 signaling and ligand scavenging functions.[2]
1.2.1 Normal Physiological Conditions
CXCR7 surface expression is extremely low in progenitor cells and lymphocytes in peripheral blood, but it can be detected in secondary lymphoid organs (such as marginal zone B cells and dendritic cells in the spleen). In addition, CXCR7 is highly expressed in the heart, nervous tissue, vascular endothelial cells, and during embryonic development. For example, in adult mice, significant expression is observed in splenic red pulp, marginal zone B cells, hippocampal and olfactory bulb neurons, and CD31⁺ vascular cells.[2]
1.2.2 Pathological Conditions
Tumors
CXCR7 is markedly upregulated in a variety of solid tumors, including breast cancer, lung cancer, renal cancer, prostate cancer, and glioblastoma, and is often associated with increased invasiveness, angiogenesis, and poor prognosis.[3] Studies have found that over 30% of breast cancer samples exhibit high CXCR7 expression, with even higher expression in tumor vasculature, which is significantly correlated with both overall survival and lung metastasis-free survival.[4] In glioblastoma patients, high CXCR7 expression is associated with significantly poorer prognosis (HR ~2.8), reduced late-stage survival under conventional treatment, and detectable CXCR7-positive staining in both tumor and vascular tissues, whereas normal brain tissue shows negligible CXCR7 expression.
Inflammation and Cardiovascular Diseases
In patients with acute coronary syndrome, platelet surface CXCR7 expression is upregulated and associated with reduced ADP- and TRAP-induced aggregation responses, suggesting a potential antithrombotic role. Pharmacological activation of ACKR3 (e.g., with the small molecule VUF11207) can induce platelet internalization and downregulate aggregation, showing a distinct signaling profile compared to CXCL12.[5]
Autoimmunity and Inflammatory Pathology
CXCR7 expression is significantly increased in models of autoimmune diseases such as rheumatoid arthritis, inflammatory bowel disease, and experimental autoimmune encephalomyelitis (EAE). Its expression can be induced by cytokines such as IL-1β, CXCL8, and LPS, contributing to the regulation of the inflammatory microenvironment and immune cell migration.[2]
2. CXCR7-Mediated Signaling
CXCR7 primarily activates intracellular signaling through β-arrestin recruitment, triggering pathways such as ERK and Akt to promote cell survival and migration. Due to its multiple ligands, CXCR7 can elicit downstream effects through various mechanisms, particularly in relation to the CXCL12/CXCR4/CXCR7 signaling axis.
One of the earliest discovered functions of CXCR7 is its “scavenger” activity, regulating the migratory capacity of CXCR4-expressing cells: upon chemokine binding, CXCR7 undergoes internalization and directs the ligand to lysosomal degradation.[6] This allows CXCR7 to modulate extracellular CXCL12 concentrations, thereby influencing CXCL12/CXCR4-mediated signaling. Within the CXCL12/CXCR4/CXCR7 axis, CXCR7 often forms heterodimers with CXCR4 to modify its signaling, either enhancing or interfering with CXCR4-mediated chemotaxis.
In models such as breast cancer, this axis regulates the ERK-PKM2-β-arrestin signaling network, inducing tumor cell metabolic reprogramming towards enhanced glycolysis and promoting proliferation and invasion. Compared with CXCR4, CXCR7 plays a more dominant role in driving this metabolic effect.
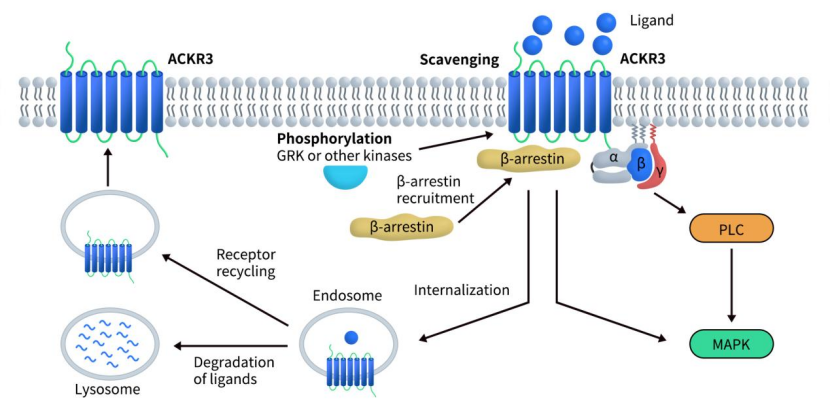
Fig 2. Schematic representation of signaling pathways for CXCR7(ACKR3).[6]
3. CXCR7-Related Diseases and Current Status of Drug Development
3.1 CXCR7-Related Diseases
Tumors
CXCR7 is highly expressed in various solid tumors, including breast cancer, liver cancer, lung cancer, prostate cancer, and gliomas. High expression is often associated with increased tumor aggressiveness, enhanced metastatic potential, and poor prognosis. Studies have shown that CXCR7 promotes tumor cell proliferation, anti-apoptotic activity, and angiogenesis, while also facilitating tumor immune evasion by modulating the immune microenvironment.[3]
Cardiovascular Diseases
In models of myocardial infarction and ischemia-reperfusion injury, CXCR7 exerts protective effects on cardiomyocytes by inhibiting apoptosis and promoting tissue repair. It also plays a key regulatory role in cardiac development, particularly in endothelial–cardiomyocyte interactions.[2.3]
Central Nervous System Diseases
CXCR7 expression is upregulated in multiple sclerosis, Alzheimer’s disease, and during stroke recovery, potentially influencing neuronal survival, glial cell activation, and blood–brain barrier permeability.[2]
Inflammatory and Autoimmune Diseases
By regulating immune cell migration and cytokine expression, CXCR7 is considered a potential therapeutic target in diseases such as rheumatoid arthritis, inflammatory bowel disease, and psoriasis.[2]
3.2 Current Status of Drug Development
As an emerging target, CXCR7 has entered drug development pipelines for multiple indications. The main therapeutic approaches include small-molecule antagonists, monoclonal antibodies, and signaling interference agents.
Small-Molecule Antagonists
- CCX771, developed by ChemoCentryx, has been shown to significantly alleviate multiple sclerosis symptoms in an experimental autoimmune encephalomyelitis (EAE) mouse model, inhibit glioma proliferation, and improve survival rates. It also reduces VLDL levels in high-fat diet models, alleviating atherosclerosis. However, under certain conditions, this molecule has also demonstrated β-arrestin recruitment activity, raising debate over its antagonist/agonist properties.
- ACT-1004-1239 is an orally active CXCR7 antagonist with optimized pharmacokinetic properties, capable of antagonizing CXCL11/12-induced β-arrestin recruitment. In dosing models, it significantly increases circulating CXCL12 levels and is progressing towards clinical evaluation.
Monoclonal Antibodies
- X7Ab is a functional neutralizing antibody that inhibits tumor growth and metastasis by blocking the interaction between CXCR7 and its ligands. It has demonstrated notable anti-tumor activity in animal models, although detailed structural or clinical data have not been disclosed in publicly available literature.
Combination Strategies
Given the synergistic functions of CXCR4 and CXCR7, dual antagonism of CXCR4 (e.g., AMD3100) and CXCR7 may offer superior therapeutic benefits in cancer treatment and stem cell mobilization, representing a promising research direction.
The development of CXCR7-targeted therapies is still at the preclinical and early clinical stages, and no marketed drugs are currently available. Furthermore, CXCR7’s atypical GPCR signaling mechanisms, expression specificity, and complex β-arrestin signaling network pose challenges for drug evaluation and model development.
4. CXCR7 Target-Related Products
As a member of the GPCR family, CXCR7 exhibits highly dynamic structural characteristics and relatively short intracellular loops, making traditional expression and purification methods insufficient for functional studies and drug screening.
To address this challenge, we have successfully developed a physiologically relevant, conformationally stable full-length CXCR7-Nanodisc membrane protein product using a mammalian cell expression system combined with Synthetic Nanodisc technology. This product preserves the native structural features of the membrane protein, greatly enhancing the feasibility and accuracy of antibody binding analysis, functional validation, and small-molecule/ligand screening.
- Data Display
Human CXCR7 full length protein-synthetic nanodisc (Cat.No.FLP100095)
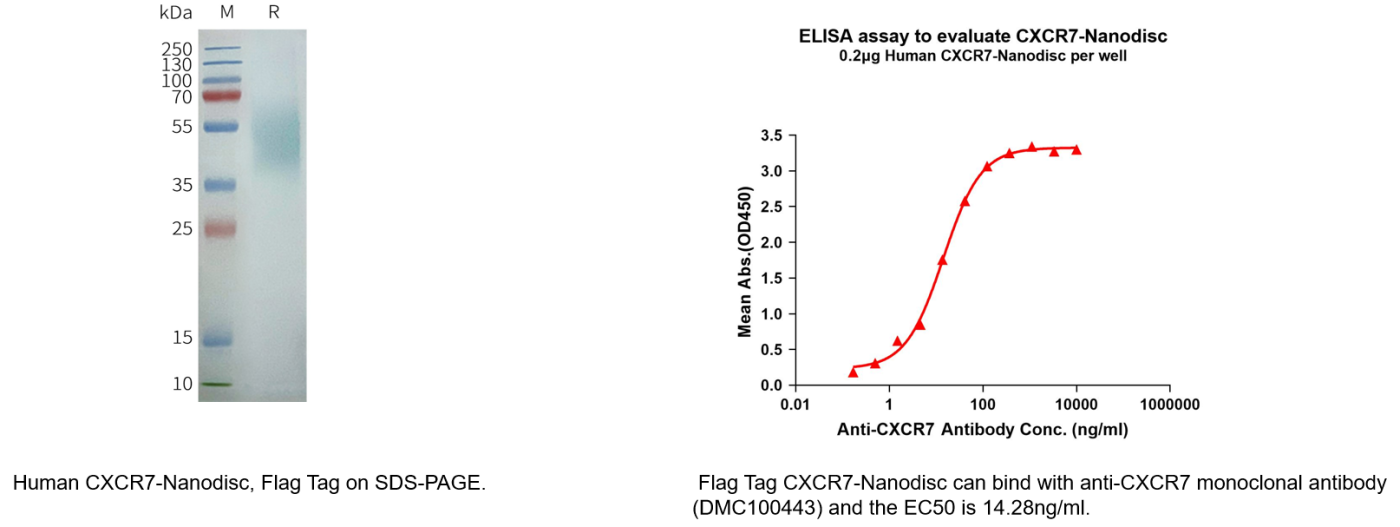
Biotinylated Human CXCR7 full length protein-synthetic nanodisc(Cat.No.FLP100095B)
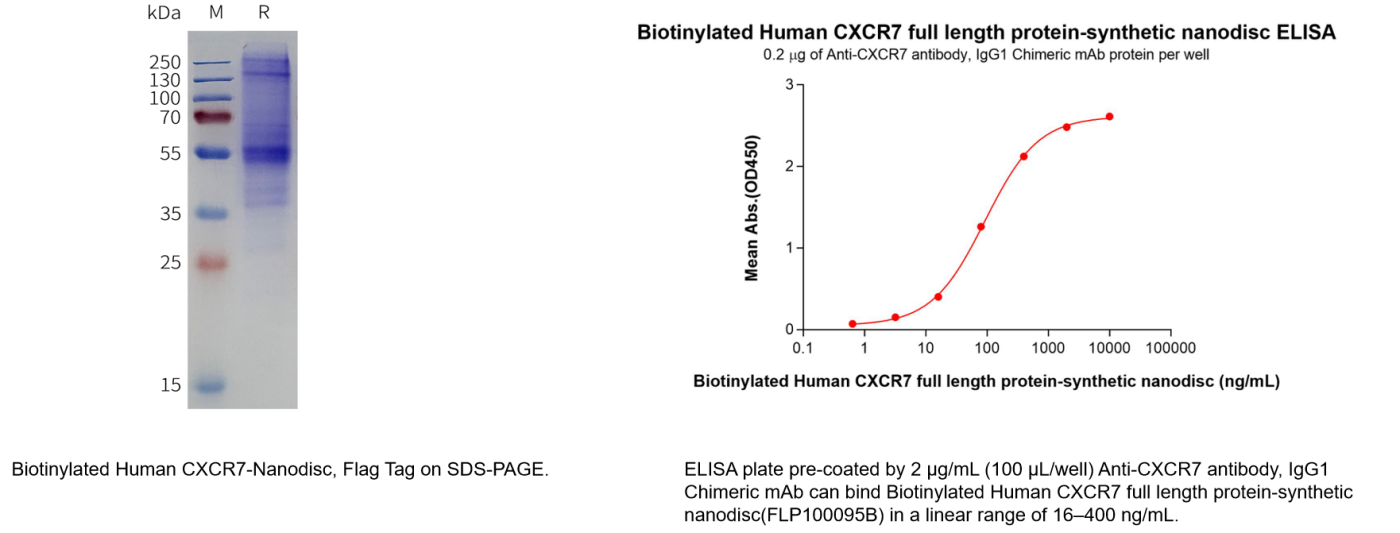
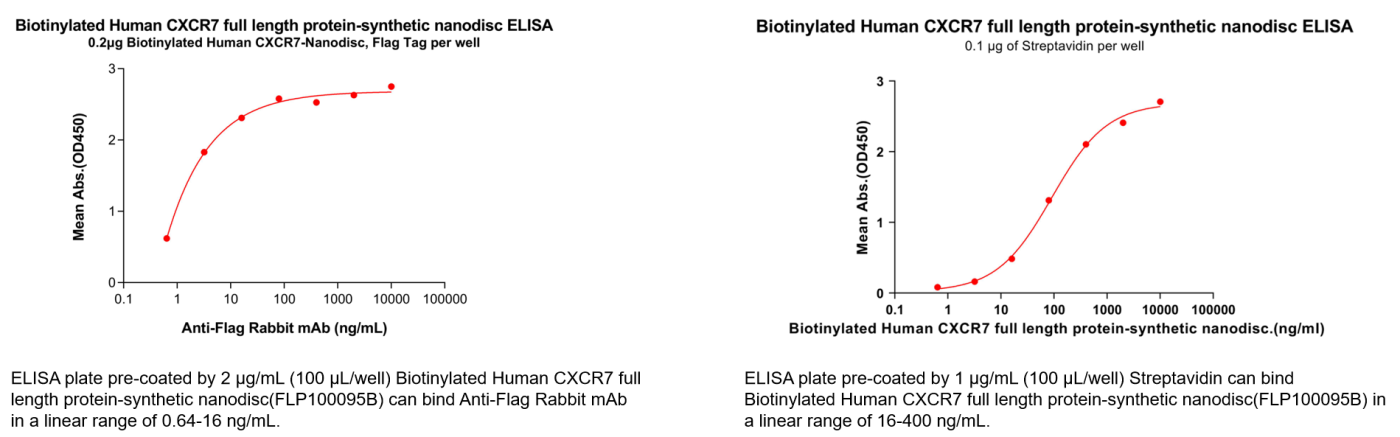
In addition, we offer recombinant proteins and various formats of recombinant monoclonal antibodies (including unlabeled, biotin-labeled, and PE-labeled) to meet experimental needs across different stages of research and drug development. We also provide a comprehensive range of services, including custom protein/antibody production, antibody humanization, affinity maturation, and stable cell line generation. Furthermore, we have established a CXCR7-targeted B cell seed library, enabling us to rapidly identify lead antibody candidates within 20 days according to customer requirements. For more details, please feel free to contact us at +1 978-912-0878.
- CXCR7-Related Product Catalog
| Product Type | Catalog No. | Product Name |
| Recombinant Protein | PME100228 | Human CXCR7 Protein, hFc Tag |
| Full-Length Membrane Protein | FLP120095 | Human CXCR7-Strep full length protein-synthetic nanodisc |
| FLP100095 | Human CXCR7 full length protein-synthetic nanodisc | |
| FLP100095B | Biotinylated Human CXCR7 full length protein-synthetic nanodisc | |
| Recombinant Monoclonal Antibody | DMC100443 | Anti-CXCR7 antibody(DMC443); IgG1 Chimeric mAb |
| DMC100443B | Biotinylated Anti-CXCR7 antibody(DMC443); IgG1 Chimeric mAb | |
| DMC100443P | PE-conjugated Anti-CXCR7 antibody(DMC443); IgG1 Chimeric mAb |
- Progress of Lead Molecules Targeting CXCR7
Reference
1. Szpakowska, M., et al., Mutational analysis of the extracellular disulphide bridges of the atypical chemokine receptor ACKR3/CXCR7 uncovers multiple binding and activation modes for its chemokine and endogenous non-chemokine agonists. Biochem Pharmacol, 2018. 153: p. 299-309.
2. Garcia-Cuesta, E.M., et al., The Role of the CXCL12/CXCR4/ACKR3 Axis in Autoimmune Diseases. Front Endocrinol (Lausanne), 2019. 10: p. 585.
3. Wang, C., W. Chen, and J. Shen, CXCR7 Targeting and Its Major Disease Relevance. Front Pharmacol, 2018. 9: p. 641.
4. Behnam Azad, B., et al., Targeted Imaging of the Atypical Chemokine Receptor 3 (ACKR3/CXCR7) in Human Cancer Xenografts. J Nucl Med, 2016. 57(6): p. 981-8.
5. Cebo, M., et al., Platelet ACKR3/CXCR7 favors antiplatelet lipids over an atherothrombotic lipidome and regulates thromboinflammation. Blood, 2022. 139(11): p. 1722-1742.
6. Duval, V., et al., Emerging Roles of the Atypical Chemokine Receptor 3 (ACKR3) in Cardiovascular Diseases. Front Endocrinol (Lausanne), 2022. 13: p. 906586.