Triggering receptor expressed on myeloid cells 2 (TREM2) has rapidly emerged as a prominent therapeutic target in neurodegenerative diseases. Since landmark studies published in The New England Journal of Medicine and Nature in 2013 demonstrated that rare missense variants in TREM2, such as R47H, markedly increase the risk of Alzheimer’s disease (AD), TREM2 has become a central focus of neuroimmunology research.
In recent years, TREM2 research has moved beyond basic biology toward clinical translation. The first-in-class oral small-molecule TREM2 agonist VG-3927 has completed Phase 1 clinical dosing with favorable safety and target engagement profiles and is advancing toward Phase 2 development. In contrast, the monoclonal antibody iluzanebart (VGL-101) failed to demonstrate efficacy in a Phase 2 trial and was subsequently discontinued, underscoring both the therapeutic potential and the challenges of targeting this pathway.
In this context, this review provides an integrated overview of TREM2, including its structure, expression, biological functions, disease relevance, and the current landscape of TREM2-targeted drug development, with the aim of clarifying its value as an emerging therapeutic target.
1. TREM2 and the TREM Family
TREM2 is a single-pass type I transmembrane receptor belonging to the immunoglobulin superfamily (IgSF) and is a member of the triggering receptor expressed on myeloid cells (TREM) family. This receptor family was first identified in 2000 and is encoded by genes clustered near the major histocompatibility complex (MHC) region. TREM family members can be broadly classified into activating and inhibitory receptors. Reported members include TREM1 (CD354), TREM2, TREM3, TREM4, plasmacytoid dendritic cell-associated TREM (pDC-TREM), and TREM-like transcripts (TLT-1 and TLT-2). Among them, TREM2 is generally regarded as a regulator of immune suppression and tissue homeostasis, and has attracted increasing attention due to its pivotal role in central nervous system (CNS) disorders.
2. TREM2 Protein Structure
The human TREM2 gene is located on chromosome 6p21.1, spans approximately 4.7 kb, and consists of five exons, encoding a 230aa protein. TREM2 is a canonical type I single-pass transmembrane IgSF receptor, originally cloned as a homolog of TREM1. The protein structure of TREM2 can be divided into four regions: a N-terminal signal peptide (1-18aa), an extracellular domain (19-173aa), a single transmembrane helix (174–195aa), and short cytoplasmic tail (196-230aa).
The extracellular domain contains a V-type immunoglobulin-like (IgV) domain responsible for binding diverse ligands, including phospholipids, apolipoprotein E (APOE), amyloid-β oligomers, and apoptotic cell debris. A short stalk region enriched in O-glycosylation sites serves as the primary cleavage site for ADAM10 and ADAM17, generating soluble TREM2 (sTREM2). The transmembrane region harbors a positively charged lysine residue, enabling non-covalent association with the adaptor protein DAP12 (TYROBP), which contains a negatively charged aspartate residue. The short cytoplasmic tail of TREM2 lacks intrinsic signaling capability and relies entirely on DAP12-mediated immunoreceptor tyrosine-based activation motif (ITAM) signaling.
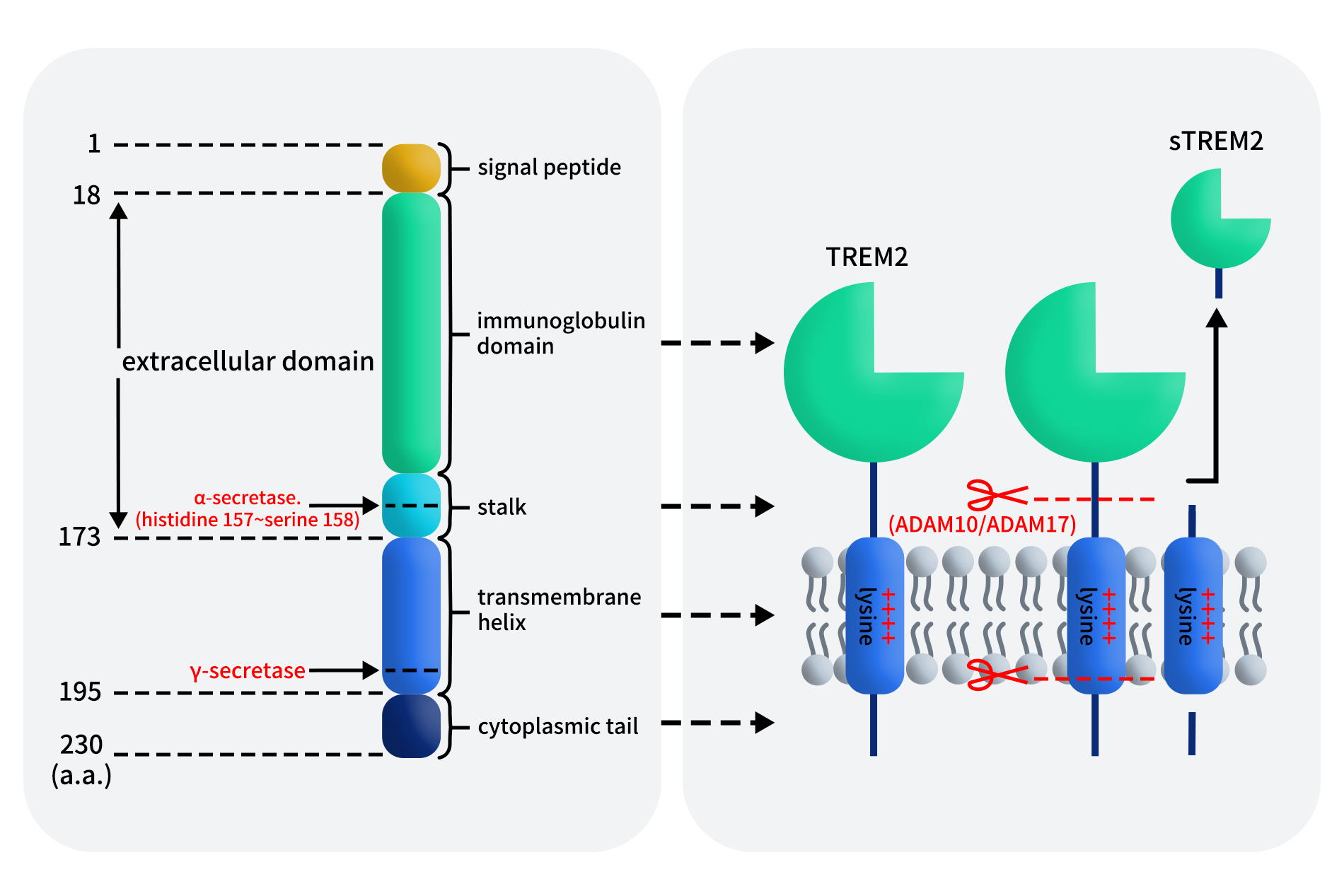
Figure 1. Schematic representation of TREM2 structure [1]
In addition to its membrane-bound form, TREM2 undergoes ectodomain shedding to generate sTREM2, primarily via ADAM10/17 cleavage at amino acid positions 157-158aa, followed by further intramembrane processing by γ-secretase. Whether additional proteases contribute to this process remains under investigation.
3. Expression Pattern and Biological Functions of TREM2
TREM2 is predominantly expressed in myeloid lineage cells. Within the CNS, its expression is most prominent in microglia, while in peripheral tissues it is detected in macrophages, monocytes, and osteoclasts. Given the central roles of these cells in innate immunity, inflammatory responses, and tissue homeostasis, TREM2 is considered a key receptor linking immune sensing to functional execution.
Under physiological conditions, TREM2 regulates myeloid cell responses to microenvironmental cues, supporting cell survival, migration, and phagocytic clearance. In the CNS, TREM2 enables microglia to recognize and remove cellular debris and pathological protein aggregates, thereby maintaining neural homeostasis. In peripheral tissues, TREM2 contributes to phagocytosis, tissue remodeling, and modulation of inflammatory responses.
Importantly, TREM2 also plays a role in limiting excessive inflammation and facilitating the transition from immune activation to resolution, positioning it as a critical regulator across multiple immune-related diseases [2].
4. TREM2/DAP12 Signaling Pathway
Because TREM2 possesses only a minimal cytoplasmic tail, its signaling function depends entirely on association with DAP12. Structural pairing between a positively charged residue in the TREM2 transmembrane region and a negatively charged residue in DAP12 enables formation of a stable TREM2/DAP12 signaling complex. DAP12 exists as a disulfide-linked homodimer and contains a cytoplasmic ITAM motif, which serves as the central signaling hub.
Ligand binding to TREM2 induces phosphorylation of the DAP12 ITAM, leading to recruitment and activation of Syk kinase. This triggers downstream signaling cascades including ERK, PI3K, PLCγ, and Vav, ultimately regulating myeloid cell survival, migration, phagocytosis, and activation state. Crosstalk with CSF1R signaling further amplifies these responses, as Src kinases downstream of CSF1R can directly phosphorylate DAP12.
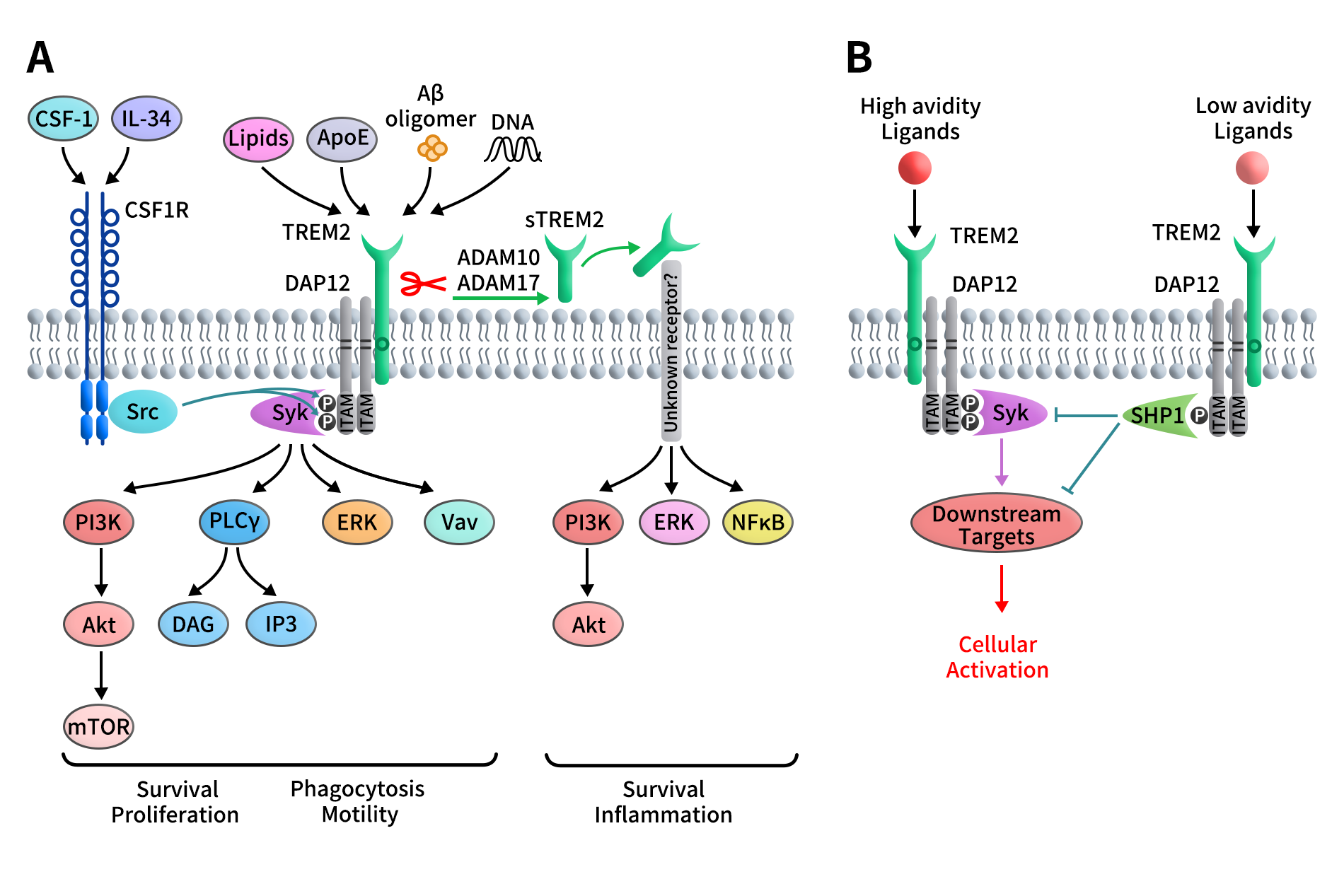
Figure 2. Schematic representation of the TREM2/DAP12 signaling pathway [3]
Notably, although ITAM motifs are classically associated with activation signals, the TREM2/DAP12 axis exhibits signal strength–dependent dual functionality. High-affinity ligand engagement induces full ITAM phosphorylation and robust Syk activation, whereas low-affinity interactions result in partial phosphorylation and recruitment of phosphatases such as SHP-1, thereby dampening immune activation. This mechanism underlies the context-dependent roles of TREM2 in immune homeostasis and inflammation.
5. TREM2 and Human Diseases
TREM2 exists in two principal forms: membrane-bound TREM2 (mTREM2) and soluble TREM2 (sTREM2). mTREM2 transduces ITAM-dependent signals via DAP12 and regulates microglial phagocytosis, inflammatory responses, and neural homeostasis. Dysfunction or genetic variants of mTREM2 impair amyloid-β clearance, accelerate pathological deposition, and exacerbate cognitive decline in Alzheimer’s disease. In other neurodegenerative disorders such as Parkinson’s disease and frontotemporal dementia, TREM2-mediated modulation of inflammation is thought to confer neuroprotection.
Although sTREM2 does not directly signal intracellularly, it serves as a biomarker of microglial activation and disease progression. Owing to the central role of mTREM2 in disease-associated microglial biology, TREM2 has become a compelling therapeutic target. Current strategies include agonistic antibodies, small-molecule modulators, and bispecific antibodies, all aimed at enhancing TREM2 function and fine-tuning microglial activation.
6. Progress in TREM2-Targeted Drug Development
TREM2 drug development currently focuses on agonistic monoclonal antibodies and small-molecule therapeutics, while additional modalities such as CAR-T cells, nanobodies, recombinant peptides, and ASOs are being explored in preclinical stages.
Globally, approximately 50 TREM2-targeted programs have been reported, with only a small fraction advancing into clinical development. These programs underscore both the strong interest in TREM2 and the inherent complexity of translating microglial biology into effective therapies.
6.1 TREM2 Monoclonal Antibodies
By early 2025, three agonistic anti-TREM2 monoclonal antibodies had entered clinical trials, led by Alector, Vigil Neuroscience, and Novartis, spanning indications from Alzheimer’s disease to rare leukodystrophies.
- AL002 (Alector)is the most advanced program, currently in Phase 2 (INVOKE-2). It originated from a strategic collaboration with AbbVie involving an upfront payment of USD 205 million. AL002 has received FDA Fast Track designation, and interim data show robust increases in cerebrospinal fluid biomarkers associated with TREM2 activation.
- Iluzanebart (VGL-101), developed by Vigil Neuroscience, targeted the IgV domain of TREM2 for the treatment of adult-onset leukoencephalopathy with axonal spheroids and pigmented glia (ALSP). Despite orphan drug and Fast Track designations, final analysis of the Phase 2 IGNITE trial showed no meaningful benefit on biomarker or clinical endpoints, leading to discontinuation of the program.
- VHB937, originally developed by Pliant Therapeutics and licensed by Novartis in a deal valued at up to USD 1.12 billion, is a high-affinity agonistic antibody designed to stabilize membrane TREM2 and prevent ectodomain shedding. Phase 2 trials have expanded into ALS and other neurodegenerative diseases.
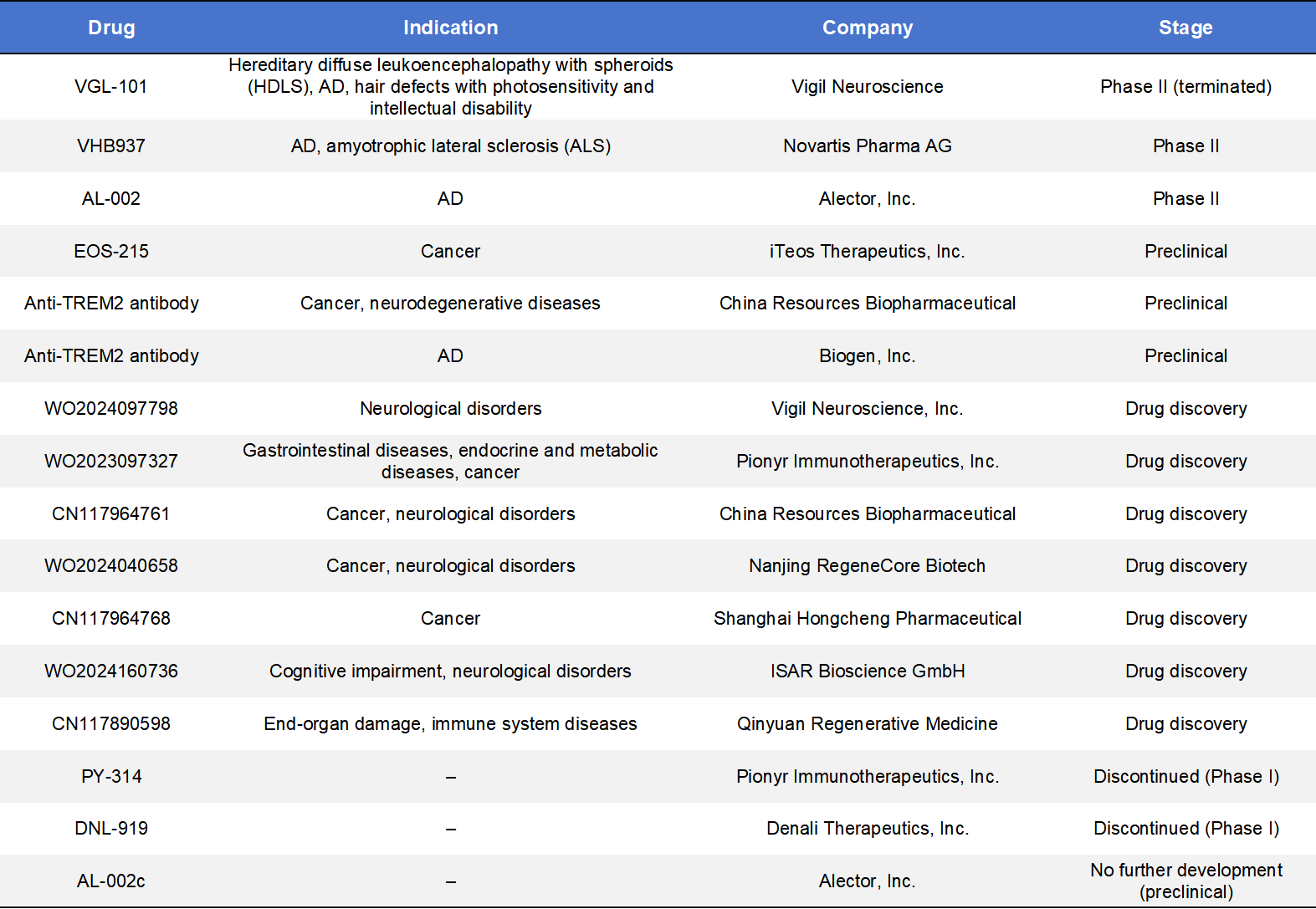
6.2 TREM2 Small-Molecule Therapeutics
Small-molecule TREM2 agonists have recently entered clinical development, offering advantages in oral dosing and blood–brain barrier penetration.
- MNA-001 (Muna Therapeutics)initiated Phase 1 trials in early 2025 for early Alzheimer’s disease and received USD 1 million in funding from the Alzheimer’s Association to support biomarker validation.
- VG-3927 (Vigil Neuroscience)represents the first oral small-molecule TREM2 agonist to enter clinical trials. In May 2025, Sanofi acquired this program for approximately USD 470 million. Phase 1 data demonstrated excellent safety and target engagement, positioning VG-3927 as a potential competitor to antibody-based approaches.
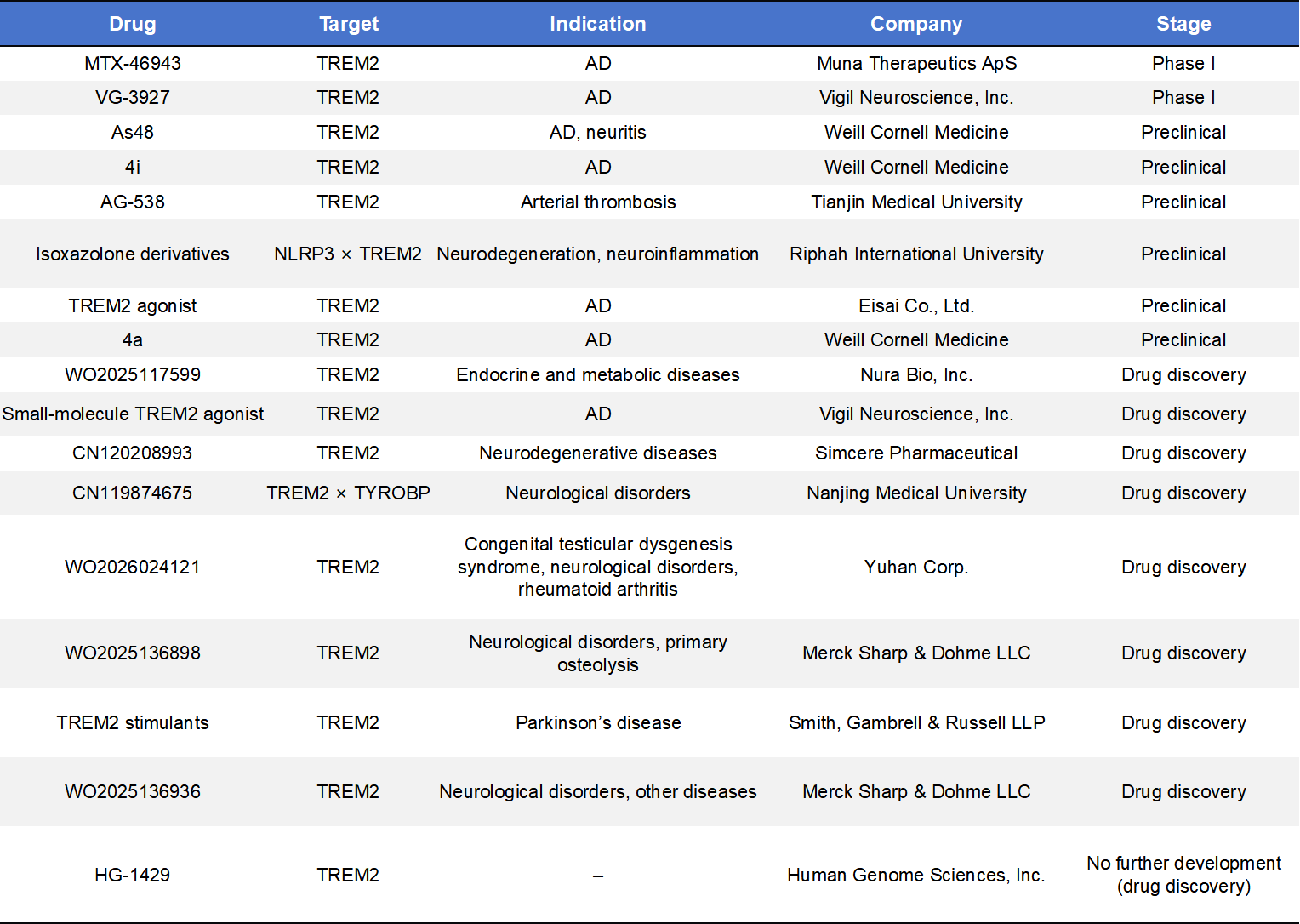
6.3 Emerging TREM2–Targeting Strategies
Beyond antibodies and small molecules, TREM2 is attracting interest from diverse therapeutic platforms, including bispecific antibodies, CAR-T therapies, nanobodies, gene therapies, and diagnostic radiotracers. These approaches aim to overcome limitations such as poor brain penetration or limited target specificity, and represent the next phase of innovation in the TREM2 field.
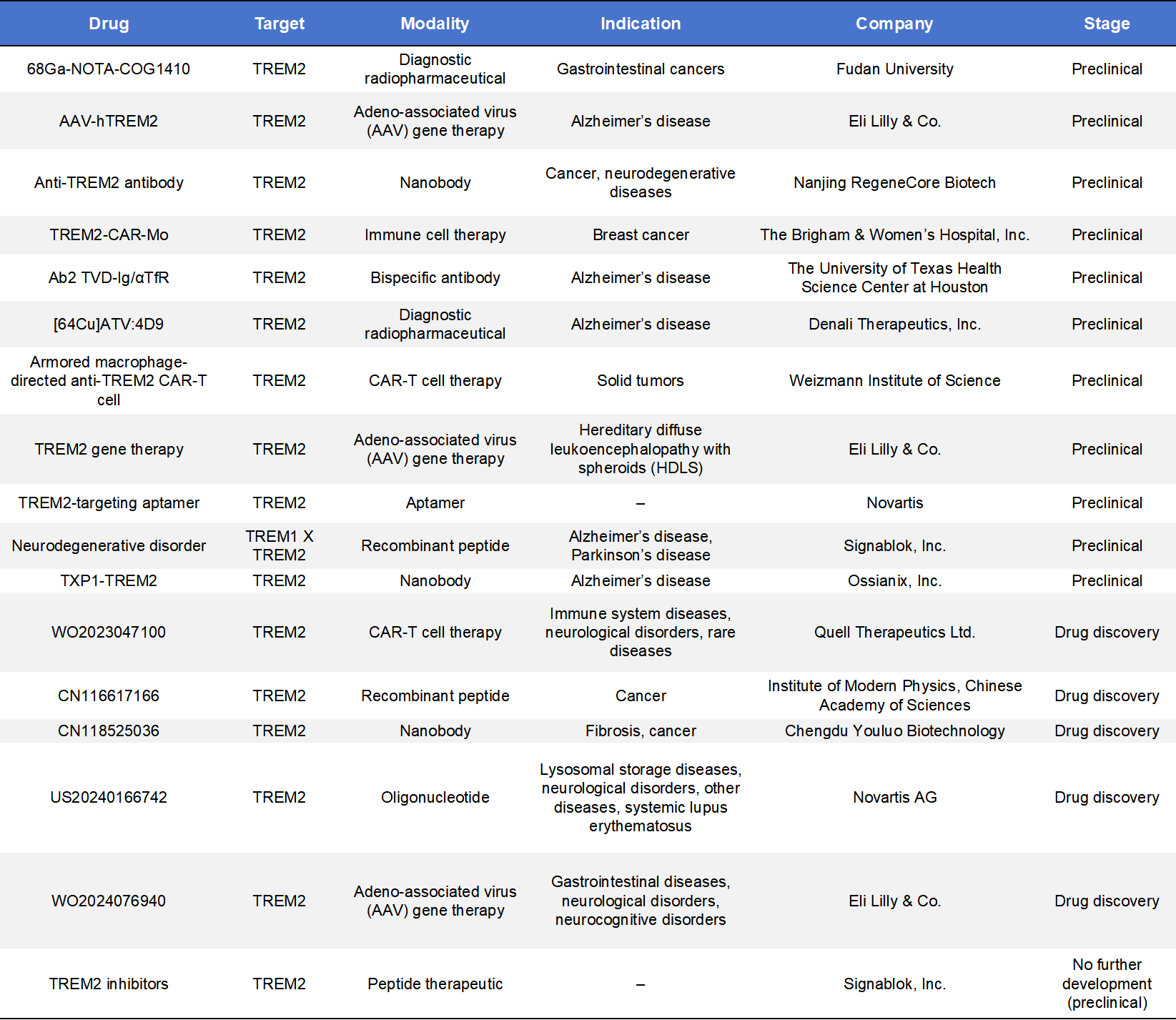
7. Dimabio TREM2 Products Supporting Drug Discovery
TREM2 has evolved from a genetic risk factor into a validated yet challenging therapeutic target. Key unresolved questions include optimal dose control to avoid excessive activation and the precise roles of mTREM2 versus sTREM2 across disease stages.
DIMA BIOTECH has developed a comprehensive portfolio of TREM2 research reagents, including full-length TREM2 proteins, multi-species recombinant TREM2 extracellular domains, flow cytometry antibodies, and reference antibodies for immunization, antibody screening, binding assays, and cell-surface expression analysis. Leveraging its proprietary single B-cell antibody discovery platform, Dimabio has also generated multiple TREM2 antibody lead candidates and welcomes collaboration opportunities for further development and validation.
| Product Type | Cat No. | Product Name |
| The full-length membrane protein | FLP100507 | Human TREM2 full length protein-synthetic nanodisc |
| ECD protein | PME100633 | Human TREM2 Protein, hFc Tag |
| PME-C100024 | Cynomolgus TREM2 Protein, hFc Tag | |
| PME-M100080 | Mouse TREM2 Protein, hFc Tag | |
| FC-validated mAb | DMC100223 | Anti-TREM2 antibody(DMC223); IgG1 Chimeric mAb |
| DMC100223P | PE-conjugated Anti-TREM2 antibody(DMC223); IgG1 Chimeric mAb | |
| DMC100223B | Biotinylated Anti-TREM2 antibody(DMC223); IgG1 Chimeric mAb | |
| BME100294 | Anti-TREM2(AL002 biosimilar) mAb | |
| BME100533B | Biotinylated Anti-TREM2(iluzanebart biosimilar) mAb | |
| BME100533 | Anti-TREM2(iluzanebart biosimilar) mAb |
Reference:
[1] Qiu H, Shao Z, Wen X, Jiang J, Ma Q, Wang Y, Huang L, Ding X, Zhang L. TREM2: Keeping Pace With Immune Checkpoint Inhibitors in Cancer Immunotherapy. Front Immunol. 2021 Sep 3;12:716710. [2] Medd M. TREM2 in Regulating Macrophage Inflammatory Responses and Disease Pathogenesis. Crit Rev Immunol. 2025;45(2):15-24. [3] Konishi H, Kiyama H. Microglial TREM2/DAP12 Signaling: A Double-Edged Sword in Neural Diseases. Front Cell Neurosci. 2018 Aug 6;12:206.