Membrane proteins are molecules that are critically important for life, undertaking key functions such as signal transduction, material transport, and cell recognition. With the deepening of drug development and structural biology research, membrane proteins have become central targets for new drug discovery and mechanistic studies. However, due to their structural complexity and poor stability, their expression and purification remain major challenges for the scientific community. This demand has also driven the rapid growth of specialized membrane protein manufacturers and high-quality membrane protein production services.
1. Membrane Proteins: The Signal Gate of Life
Membrane proteins are core components of the cell membrane, responsible for signal transmission, molecule transport, and cellular communication – often referred to as the “signal gate” of life. In biomedical research, because they directly participate in cell signaling regulation and drug binding, membrane proteins are among the most valuable targets in drug discovery and disease research. Statistically, more than 60% of approved drugs target membrane proteins, covering several important families such as GPCRs (G protein–coupled receptors), ion channels, and transporters [1].
The uniqueness of membrane proteins lies in their function being highly dependent on their three-dimensional structure and lipid membrane environment. This dependency introduces severe challenges in in vitro studies, expression, and stabilization. Researchers often need to maintain the native conformation while achieving high purity and high activity in their expressed products – placing high demands on the stability of the experimental system and accumulation of technical expertise.
2. Types and Functions of Membrane Proteins
Membrane proteins, as important components of the cell membrane, can be divided into two major categories based on how they associate with the membrane and their structural features: integral (or embedded) membrane proteins and peripheral membrane proteins.
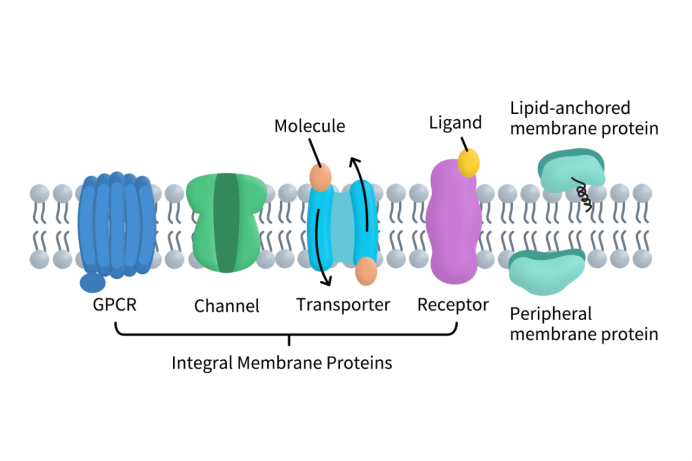
Figure 1. The Types of Membrane Proteins
Integral membrane proteins typically traverse the lipid bilayer and contain one or multiple transmembrane domains. They are the core players in signal transduction, material transport, and receptor recognition. Peripheral membrane proteins, on the other hand, attach to the membrane surface. Through interactions with lipids or integral proteins, they participate in forming signaling complexes, anchoring to the cytoskeleton, and regulating membrane protein localization [2].
Among integral membrane proteins, there are three functional families that are particularly critical in research and drug development:
- GPCRs (G protein–coupled receptors): These proteins sense a variety of external signals — such as hormones, neurotransmitters, and light — and convey information into the cell, regulating immune, neural, metabolic, and cardiovascular processes. GPCRs are also core drug targets, accounting for about one-third of approved drug targets.
- Ion Channels: By regulating the transmembrane flow of ions (e.g., sodium, potassium, calcium), these channels participate in neural signaling, muscle contraction, and heartbeat regulation. They are commonly studied in cardiovascular and neurological disease research and drug screening.
- Transporters: Responsible for translocating molecules and ions across membranes, transporters help maintain cellular homeostasis. They also directly influence drug absorption, distribution, and efflux, making them critical in studying resistance and metabolic diseases.
Although peripheral membrane proteins do not span the membrane, they remain indispensable in cell signaling regulation. For example, they can serve as regulatory factors of signaling complexes or as cytoskeletal anchor points; they are involved in receptor activation, signal transmission, and positioning of membrane proteins.
3. Applications of Membrane Proteins in Scientific Research and Drug Development
Membrane proteins are not only central to fundamental biological research, but also indispensable targets in modern drug development:
- Antibody drug development: Many antibody drug targets are located on the cell surface, such as immune checkpoint proteins (PD-1, PD-L1, CTLA-4) and clinically important tumor targets (e.g., HER2, EGFR, Claudin18.2, TROP2). Many of these are multi-pass membrane proteins whose native conformation is critical for antibody binding. Thus, high-activity, high-purity membrane proteins are needed as immunogens, screening materials, or functional validation tools. Membrane targets in hematological cancers (e.g., CD20, CD19, CD22), immune regulation (e.g., OX40, 4-1BB, CD47, SIRPα), and inflammation/virus-related diseases (e.g., CCR5, CXCR4) also rely on stable membrane protein samples for antibody selection and affinity optimization.
- Small-molecule drug screening: GPCRs, ion channels, and transporters are some of the most important small-molecule drug targets. But their strong hydrophobicity and dependence on membrane environments mean that traditional detergent-based systems often distort their conformation, affecting binding experiments. Nanodisc technology, by reconstituting the membrane protein into a lipid bilayer-like environment, allows them to exist in a more physiologically relevant form. As a result, nanodiscs are widely used in high-throughput screening (HTS), ligand binding kinetics, drug binding-pocket analysis, and studies of GPCR–ligand coupling mechanisms.
Nanodiscs are especially suitable for classic GPCRs (like β2AR, CCR5, CXCR4), TRP channels, sodium/potassium/calcium ion channels, etc., making small-molecule screening more accurate and reproducible. This has become a key technological trend in membrane protein drug discovery in recent years.
- Structural biology & mechanistic research: Membrane proteins are focal points in structural biology. With the rapid development of cryo-EM (cryo–electron microscopy), high-resolution structures of multi-pass targets such as GPCRs, transporters, and ion channels have been increasingly resolved. But these studies depend heavily on high-purity, stable, correctly folded membrane protein samples. Beyond cryo-EM, membrane proteins are also used for SPR/BLI, microscale thermophoresis (MST), lipid reconstitution platforms, and complex assembly analyses (e.g., GPCR–G-protein complexes, regulatory complexes of ion channels) [3].
For example, protein-protein or protein–ligand interaction studies help reveal binding interfaces; MST is used to measure binding affinities; lipid platforms allow investigation of how the membrane environment regulates activity. These applications highlight that only high-quality membrane protein samples enable deep insight into signaling mechanisms, drug-binding modes, and conformational activation — thereby accelerating structure-driven drug design (SBDD).
4. Core Challenges in Membrane Protein Research & Production, and Proposed Solutions
Despite their irreplaceable importance in drug development and life sciences, membrane proteins face multiple challenges in research and manufacture:
- Structural complexity: Multi-pass transmembrane helices and hydrophobic regions make it easy for the protein to misfold or aggregate during in vitro expression, leading to loss of activity.
- Membrane environment dependence: The native lipid membrane is crucial to function, but traditional soluble expression systems cannot mimic the bilayer environment, making it difficult to preserve stability and biological activity.
- Low expression and difficult purification: Membrane proteins often have low yield; purification is complex, sensitive to detergents and buffer conditions, which makes reproducibility and yield low. These issues increase cost and slow down research and drug development.
To address these challenges, research groups and companies usually adopt a combination of strategies:
- Selecting suitable host expression systems (e.g., mammalian or insect cells) to achieve correct post-translational modifications
- Optimizing gene sequences and expression conditions to boost yield
- Using detergent screening, nanodiscs, or liposome reconstruction to maintain native conformation
- Establishing rigorous QC systems (e.g., activity assays, structural checks) to verify folding and function
- Utilizing professional membrane protein production services to improve success rates, obtaining stable, active, and reproducible material for antibody screening, small-molecule development, and structural biology.
In particular, more and more institutions are integrating multiple technologies into systematic, reusable membrane-protein production workflows to reduce trial-and-error costs and improve consistency across projects. Among these, the combination of mammalian-cell expression + nanodisc reconstitution is becoming a trend – preserving native conformation while accommodating complex multi-pass targets (like GPCRs and ion channels).
5. DIMA BIOTECH: A Professional Membrane Protein Production Solution
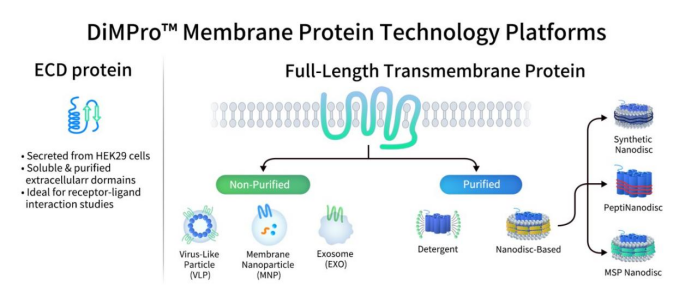
DIMA BIOTECH is a biotech company specializing in preclinical R&D products and services for druggable targets. Given their focus on druggable targets, the challenges of preparing multi-pass membrane proteins were essential hurdles to overcome. Based on these challenges and application demands, DIMA has, after extensive development, established the DiMPro™ Functional Membrane Protein Development Platform, which is based on mammalian cell expression. This platform specializes in the production of membrane proteins, especially multi-pass proteins, with the goal of providing high-quality solutions for both antibody and small-molecule drug development.
For single-pass membrane proteins, they mainly use an extracellular-domain (ECD) fusion expression system. For full-length multi-pass proteins (such as GPCRs and Claudins), they deploy a seven-technology full-length multi-transmembrane protein expression platform, which includes a purified protein platform and a non-purified platform.
- Purified protein platform: includes Synthetic Nanodisc, MSP Nanodisc, PeptiNanodisc, and detergent-based systems.
- Non-purified platform: includes MNP (membrane nanoparticle), VLP (virus-like particle), and exosome platforms.
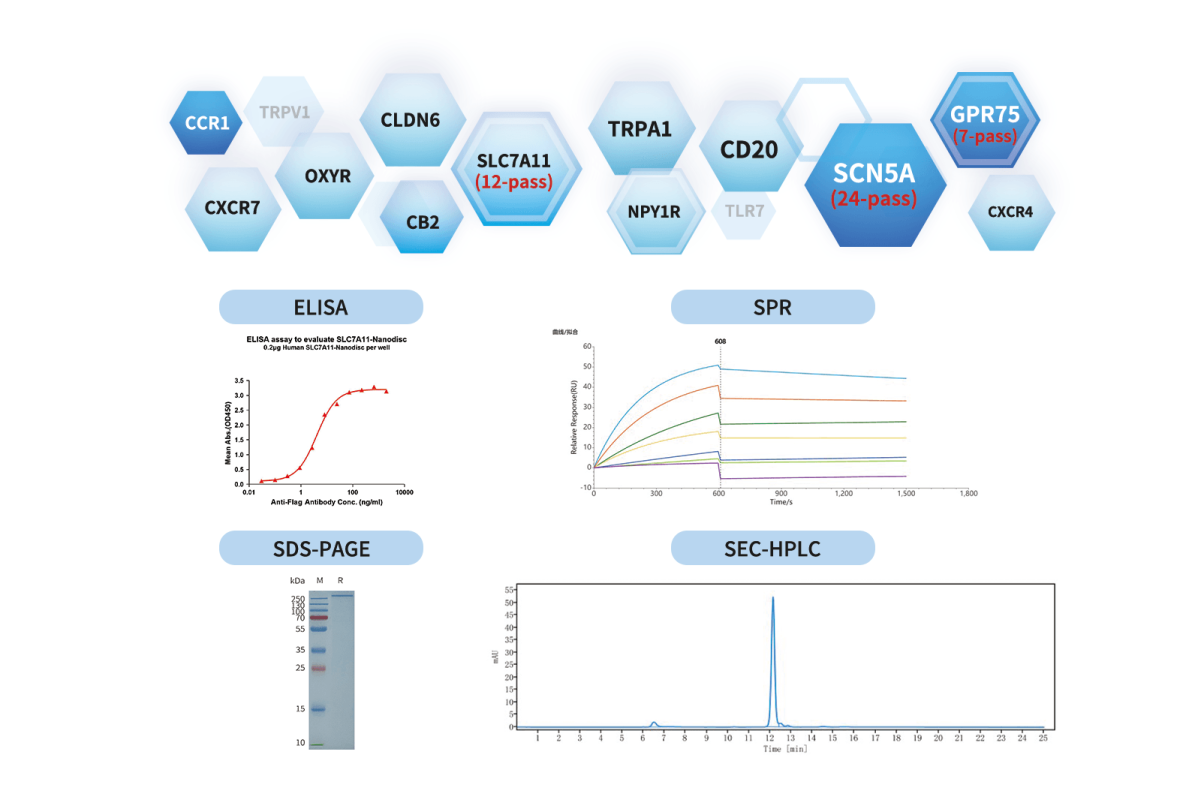
DIMA BIOTECH, leveraging its mature expression platform and rich project experience, has produced more than 900 full-length membrane proteins, covering over 700 GPCRs, ion channels, and other hot targets, with maximum transmembrane count reaching 24. They also implement a strict quality-control system (including SDS-PAGE, SEC-MALS, activity assays, etc.) to ensure the structural integrity and biological activity of the membrane proteins.
Reference:
[1] Overington JP, et al. How many drug targets are there? Nat Rev Drug Discov. 2006.
[2] Lodish H, et al. Molecular Cell Biology. 9th edition, W.H. Freeman, 2021.
[3] Carpenter EP, et al. Overcoming the challenges of membrane protein crystallography. Curr Opin Struct Biol. 2008.
