In March 2024, a preclinical study titled “Raludotatug Deruxtecan, a CDH6-Targeting Antibody–Drug Conjugate with a DNA Topoisomerase I Inhibitor DXd, Is Efficacious in Human Ovarian and Kidney Cancer Models” was published in Molecular Cancer Therapeutics. This study demonstrated that Raludotatug Deruxtecan, developed by Daiichi Sankyo, is effective against human ovarian and kidney cancer models. Additionally, at the 2023 ESMO Congress, the results of a Phase I trial (NCT04707248) analyzing Raludotatug Deruxtecan as a monotherapy in previously treated ovarian cancer patients were presented. The subgroup analysis showed an objective response rate of 46% and a disease control rate of up to 98%. Considering the remarkable efficacy of CDH6, let’s delve into the background and current landscape of targeted therapies in this field.
1. CDH6 and Classic Cadherins
Cadherin 6 (CDH6), also known as fetal kidney cadherin or K-cadherin, is a single-pass transmembrane protein composed of 790 amino acids. It belongs to type II classic cadherins and is one of the members of the cadherin superfamily. The cadherin superfamily consists of four main subfamilies: classic cadherins, desmosomal cadherins, protocadherins, and atypical cadherins [1]. As shown in the figure below, classic cadherins are composed of three parts: an extracellular domain, a transmembrane domain, and a cytoplasmic domain. The extracellular domain contains five cadherin repeats (EC1-EC5). The extracellular structure, particularly the N-terminal domain (EC1), plays a crucial role in the homophilic recognition between cadherin molecules.
Classic cadherins are further classified into type I and type II based on whether the first EC domain contains the histidine-alanine-valine (HAV) motif. Type I cadherins consist of only five members: E-cadherin (CDH1), N-cadherin (CDH2), P-cadherin (CDH3), M-cadherin (CDH15), and R-cadherin (CDH4); CDH5-12, CDH18-20, CDH22, and CDH24 belong to type II cadherins. In addition to differences in the HAV motif, type I and type II cadherins also differ in certain amino acid residues. The conformation of cadherin molecules is stabilized only in the presence of Ca2+. The binding of Ca2+ to the extracellular portion of the peptide chain is a prerequisite for cadherin-mediated cell-cell adhesion. Calcium-binding sites, composed of short highly conserved amino acid sequences, are located between adjacent extracellular repeat sequences [2]. The cytoplasmic domain of classic cadherins can bind to p120-catenin and β-catenin, whereas other members of the cadherin superfamily (including protocadherins) do not bind to catenins.
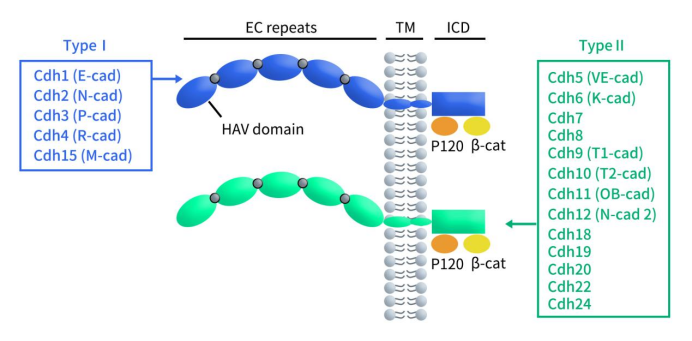
Figure 1. The structure of classical Cadherin [3]
In humans, cadherin-6 is a protein encoded by the CDH6 gene (also known as CAD6 or KCAD), located on chromosome 5. Unlike other type II classic cadherins, CDH6 contains an RGD motif in the EC1 domain and an HAV motif in the EC5 domain, which are important for stabilizing and aggregating adjacent monomers. Similar to other classic cadherins, the cytoplasmic tail of CDH6 can bind to p120-catenin and β-catenin to regulate the actin cytoskeleton. Due to the central role of β-catenin in the Wnt signaling pathway, there is a link between β-catenin signaling and CDH6-mediated cell adhesion [4].
CDH6 is involved in the morphogenesis and development of the embryonic kidney and drives the mesenchymal-epithelial differentiation necessary for kidney morphogenesis. However, CDH6 expression is very weak in adult kidneys. In normal adult tissues, CDH6 expression is restricted to renal tubules, bile duct epithelial cells, and other areas. Additionally, CDH6 is also expressed in platelets, playing a functional role in platelet aggregation and thrombus formation. This function is mediated by the binding of the RGD motif to the αIIbβ3 integrin, which undergoes a conformational change and binds to fibrinogen, leading to platelet crosslinking [5].
2. CDH6 and Tumors
CDH6 is reported to be highly expressed in thyroid cancer, gastric cancer, pancreatic cancer, and ovarian cancer, with kidney cancer being the first cancer where high expression of CDH6 was observed.
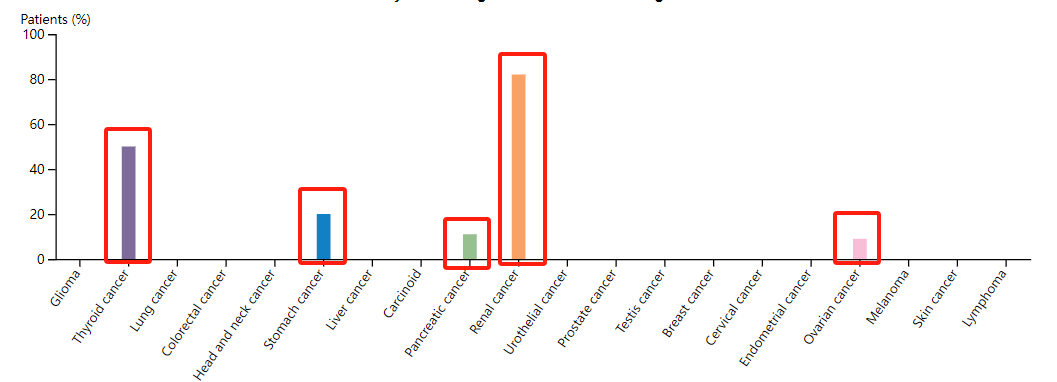
Figure 2. The expression of CDH6 in various tumors (*Data derived from The Human Protein ATLAS)
The mechanism of CDH6’s role in cancer is not fully understood, but research by Rubén A. Bartolomé and colleagues indicates that CDH6 and associated integrins play a key role in the progression of ovarian and kidney cancers. As shown in the figure below, in cells with high expression of αIIbβ3, CDH6 preferentially binds to αIIbβ3, leading to the activation of α2β1 integrin. The activation of integrins promotes cell adhesion, invasion, and proliferation, resulting in the metastatic spread of cancer cells. In cells with low expression of αIIbβ3, CDH6 and CDH17 bind to α2β1 integrin, and this cadherin/integrin interaction promotes cell adhesion, migration, invasion, and proliferation by activating the SRC, FAK, AKT, and ERK pathways.
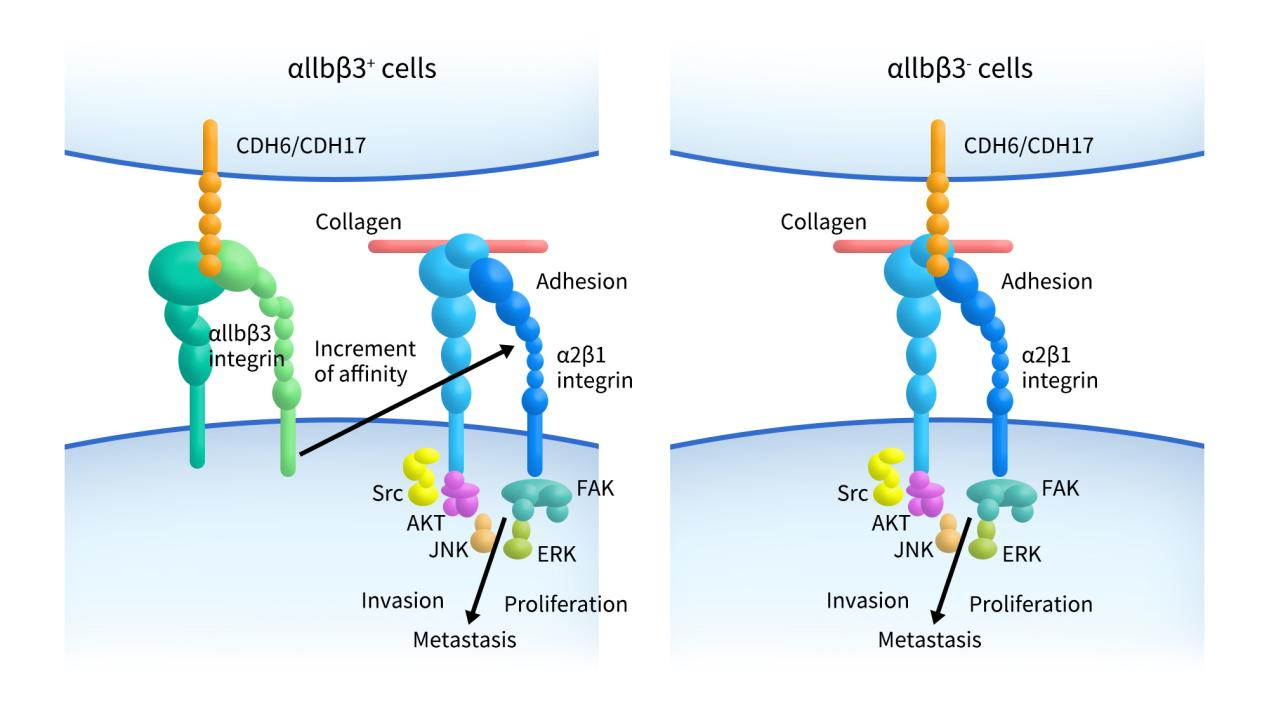
Figure 3. CDH6 and αIIb/α2 integrins are required for lung homing in ovarian and renal cancer[6]
3. Current Research Progress on CDH6 Targeted Therapy
As mentioned above, CDH6 is a type II classical member of the cadherin family, involved in cell-cell adhesion and tissue development. CDH6 shows low expression in normal adult tissues, but is highly expressed in multiple solid tumors, particularly ovarian cancer and renal cell carcinoma, where it is significantly associated with tumor invasion, metastasis, and poor prognosis. These characteristics make CDH6 a promising therapeutic target in oncology.
Based on incomplete statistics, there are currently 14 CDH6-targeted therapeutic programs worldwide, including 5 clinical-stage programs, 4 preclinical programs, 2 drug discovery-stage programs, and 2 discontinued programs. Notably, all CDH6-targeted therapies that have entered clinical development are antibody drug conjugates (ADCs).
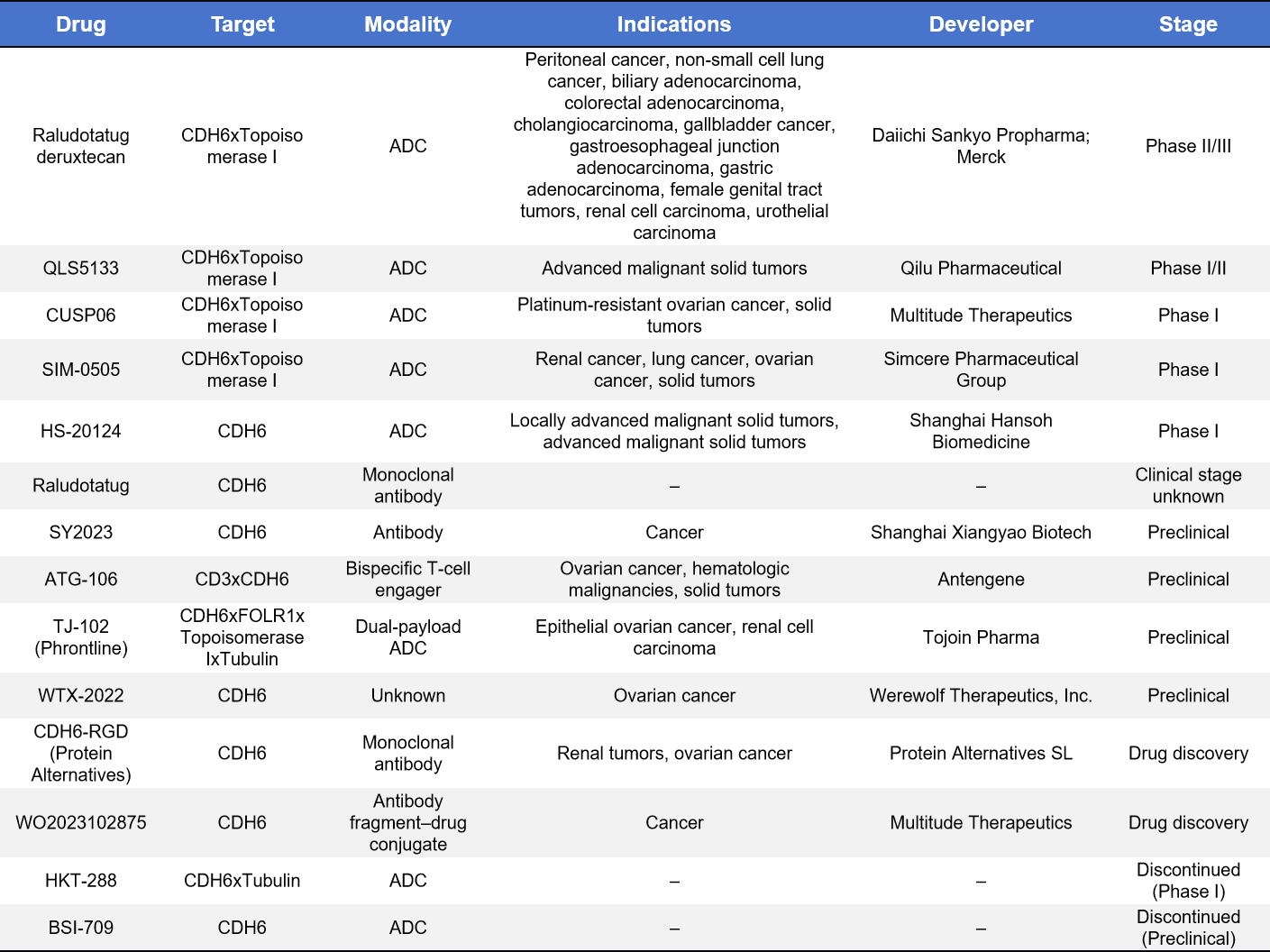
- Raludotatug deruxtecan
Raludotatug deruxtecan (also known as DS-6000 or R-DXd) is a CDH6-targeting ADC developed by Daiichi Sankyo. R-DXd consists of a humanized anti-CDH6 IgG1 monoclonal antibody, a cleavable linker, and the cytotoxic payload DXd, employing Daiichi Sankyo’s proprietary GGFG tetrapeptide linker-DXd platform, with a DAR of 8. In February 2024, R-DXd received Orphan Drug Designation from the European Union for ovarian cancer.
Early Phase I clinical data (NCT04707248) demonstrated encouraging efficacy in patients with advanced ovarian cancer, with an objective response rate (ORR) of 46%, a disease control rate (DCR) of 98%, and a median duration of response (mDoR) of 11.2 months. Daiichi Sankyo is currently collaborating with Merck to conduct a Phase II/III study (NCT06161025) in patients with platinum-resistant ovarian cancer.
- QLS5133
QLS5133 is a CDH6-targeting ADC independently developed by Qilu Pharmaceutical for the treatment of CDH6-high advanced solid tumors. It consists of a humanized anti-CDH6 monoclonal antibody conjugated via a cleavable linker to a topoisomerase I inhibitor payload (QLS6916), with a relatively high DAR of approximately 8. Upon internalization into tumor cells, QLS5133 releases its cytotoxic payload to achieve targeted tumor killing, potentially accompanied by a bystander effect.
In 2025, QLS5133 received approval from the National Medical Products Administration (NMPA, CDE) in China to initiate clinical trials and is currently in Phase I/II development. The ongoing studies aim to evaluate safety, tolerability, pharmacokinetics, and preliminary antitumor activity in patients with advanced solid tumors, including ovarian cancer, fallopian tube cancer, primary peritoneal cancer, and renal cell carcinoma. The trial has been initiated and is actively enrolling patients. Preclinical data support robust antitumor activity across multiple CDH6-positive tumor models with a manageable safety profile.
- CUSP06
CUSP06, also known as AMT-707, is a CDH6-targeting ADC developed by Multitude Therapeutics, Inc. It comprises a proprietary high-affinity anti-CDH6 antibody, a protease-cleavable linker, and the payload exatecan, a topoisomerase I inhibitor, with a DAR of 8. CUSP06 is currently in Phase I clinical development (NCT06234423) for advanced solid tumors and platinum-resistant ovarian cancer.
In June 2022, Multitude Therapeutics entered into a licensing agreement with OnCusp Therapeutics for the development and commercialization of AMT-707 (now CUSP06). Under the agreement, OnCusp obtained exclusive global rights outside Greater China, while Multitude received upfront payments, development, regulatory, and sales milestone payments, as well as tiered royalties.
Preclinical studies indicate that CUSP06 exhibits a stronger bystander effect compared with DXd-based CDH6 ADCs. It selectively binds to cell-surface CDH6 and is efficiently internalized into CDH6-positive ovarian and renal cancer cells. CUSP06 demonstrated potent antiproliferative activity in multiple CDH6-positive cancer cell lines in vitro, and tumor regressions were observed in both CDH6-low and CDH6-high patient-derived xenograft (PDX) models in vivo [7].
- SIM-0505
SIM-0505 is a CDH6-targeting ADC developed by Simcere Zaiming, incorporating a differentiated antibody epitope conjugated to a topoisomerase I inhibitor payload. It is designed to selectively target CDH6-high solid tumors such as ovarian cancer and renal cell carcinoma. Preclinical studies have demonstrated strong antitumor activity and a favorable safety profile across multiple tumor models.
SIM-0505 is currently in Phase I clinical development, with dose-escalation studies initiated in China and expansion into the United States in 2025, marking its transition into global clinical development. The ongoing trials focus on evaluating safety, tolerability, pharmacokinetics, and preliminary efficacy.
In addition, in 2025, Simcere Zaiming completed a major international licensing transaction with NextCure, granting NextCure global development and commercialization rights outside Greater China. The deal carries a total potential value of up to USD 745 million, including milestone payments and tiered royalties, significantly enhancing the global profile and commercial potential of SIM-0505.
- HS-20124
HS-20124 is a CDH6-targeting ADC candidate developed by Hansoh Pharmaceutical, featuring a DAR of 8 and a topoisomerase inhibitor payload, designed to selectively eliminate CDH6-high solid tumor cells. In preclinical studies, HS-20124 demonstrated inhibitory activity against CDH6-positive tumor cells, supporting its advancement into clinical evaluation.
HS-20124 is currently in Phase I clinical development. An open-label, multicenter Phase Ia/1b study (NCT06763159) was initiated in October 2024, including dose-escalation and dose-expansion cohorts to assess safety, tolerability, pharmacokinetics, and preliminary antitumor activity. The study is expected to enroll approximately 450 patients with advanced solid tumors and is projected to complete by the end of 2027, with tumor-specific efficacy explored in expansion cohorts.
- HKT-288
HKT-288, also known as NOV-13, is a CDH6-targeting ADC developed by Novartis Pharma AG, composed of a fully human anti-CDH6 antibody, a cleavable SPDB linker, and the cytotoxic payload DM4. The program was discontinued after Phase I clinical evaluation.
In the first-in-human study (NCT02947152), patients treated at a dose of 0.75 mg/kg reported three suspected treatment-related Grade 2 neurological adverse events, including seizure in one patient and aphasia with encephalopathy in another. As the underlying mechanism of these neurological toxicities could not be clearly determined, the study was terminated early.
In addition, preclinical-stage programs include the bispecific antibody ATG-106 (CD3xCDH6) developed by Antengene Corporation, and the CDH6 ADC BSI-709 developed by Biosion, Inc., the latter of which has been discontinued. Meanwhile, Protein Alternatives SL is developing a monoclonal antibody CDH6-RGD, which is currently at the drug discovery stage.
4. DIMA Biotech’s CDH6-Related Products
Dima Biotech is a biotechnology company focused on preclinical development products and services for druggable targets. DIMA now offers a full range of products and services related to the CDH6 target. These products include active proteins, reference antibodies, and flow cytometry-validated monoclonal antibodies. The services cover customized antibody development across various species, antibody humanization, and affinity maturation. Additionally, to accelerate the development of CDH6-based therapies, Dima has established a CDH6 single B-cell seed bank, allowing for the generation of lead antibody molecules in as little as 28 days. Currently, we have screened 26 lead molecules for CDH6, all of which are cross-reactive with both human and monkey, with 25 also showing cross-reactivity with mice. Customers can receive these molecules the next day for functional evaluation. For some molecules, we are also conducting ADC internalization activity and cytotoxicity validations. Please feel free to inquire for specific data.
- CDH6 Protein&Antibody&Stable Cell Line&CDX SlideSet
| Product type | Cat. No. | Product Name |
| Recombinant Protein | PME101111 | Human CDH6 Protein, His Tag |
| PME-C100044 | Cynomolgus CDH6 Protein, His Tag | |
| PME-M100112 | Mouse CDH6 Protein, His Tag | |
| FC-validated Antibody | DMC100675 | Anti-CDH6 antibody(DMC675); IgG1 Chimeric mAb |
| Reference Antibody | BME100232 | Anti-CDH6(raludotatug biosimilar) mAb |
| BME100234 | Anti-CDH6(AMT-707 biosimilar) mAb | |
| Biotin-labeled Antibody | BME100232B | Biotinylated Anti-CDH6(raludotatug biosimilar) mAb |
| DMC100675B | Biotinylated Anti-CDH6 antibody(DMC675); IgG1 Chimeric mAb | |
| Stable Cell Line | CEL100055 | Hu_CDH6 CHO-S Cell Line |
| CDX SlideSet | SLI100004 | Balb/C nu HuH7 DiSliceX™ SlideSet |
- Progress on CDH6 Lead mAb Molecules
Reference:
[1]Brasch, J., Harrison, O. J., Honig, B., & Shapiro, L. Thinking outside the cell: how cadherins drive adhesion. Trends in cell biology, 2012, 22(6), 299–310.
[2]Ivanov DB, Philippova MP, Tkachuk VA. Structure and functions of classical cadherins. Biochemistry (Mosc). 2001, Oct;66(10):1174-86.
[3]Basu, R., Taylor, M. R., & Williams, M. E. (2015). The classic cadherins in synaptic specificity. Cell adhesion & migration, 9(3), 193–201.
[4]Stewart DB, Barth AI, Nelson WJ. Differential regulation of endogenous cadherin expression in Madin-Darby canine kidney cells by cell-cell adhesion and activation of beta -catenin signaling. J Biol Chem. 2000 Jul 7;275(27):20707-16.
[5]Casal, J. I., & Bartolomé, R. A. Beyond N-Cadherin, Relevance of Cadherins 5, 6 and 17 in Cancer Progression and Metastasis. International journal of molecular sciences,2019,20(13), 3373.
[6]Bartolomé, R. A., Robles, J., Martin-Regalado, Á., et al. CDH6-activated αIIbβ3 crosstalks with α2β1 to trigger cellular adhesion and invasion in metastatic ovarian and renal cancers. Molecular oncology, 2021, 15(7), 1849–1865.
Wei Lu, Jing Shi, Shu-Hui Liu, Nicole Covino, Xun Meng, Eric D. Slosberg. CUSP06/AMT-707, a new CDH6-targeting antibody-drug conjugate, demonstrates potent antitumor activity in preclinical models [abstract]. In: Proceedings of the American Association for Cancer Research Annual Meeting 2023; Part 1 (Regular and Invited Abstracts); 2023 Apr 14-19; Orlando, FL. Philadelphia (PA): AACR; Cancer Res 2023;83(7_Suppl):Abstract nr 6320.
