1. The Biggest Bottleneck in Early Antibody Discovery Is Never Technology—It’s “Waiting for Lead Molecules”
In antibody drug development, early discovery is often the most time-consuming stage. Immunization, screening, validation, and sequence processing can delay a project by weeks or even months, affecting program initiation, partnership timing, and competitive positioning.
As the antibody discovery landscape becomes increasingly competitive, speed is now a core differentiator. Both established biopharma companies and emerging biotech teams are seeking faster access to ready-to-develop lead antibodies.
This is why antibody sequence transfer and licensing has become a strategic choice: by acquiring pre-validated sequences, teams can skip the lengthy discovery phase and focus resources on antibody engineering, CMC, and preclinical development.
Traditional CRO models—still based on custom projects—often require months of delivery time and can no longer meet the industry’s need for speed. What the field truly needs is an off-the-shelf, immediately available solution: validated sequences with complete data packages and clear IP paths.
DIMA BIOTECH has built precisely such a platform. By converting years of discovery and engineering experience into a true off-the-shelf lead antibody sequence library, DIMA provides transfer and exclusive licensing options that enable R&D teams to “start today and move immediately.”
2.Why Antibody Sequence Transfer Is the Best Choice for Faster R&D
1) Dramatically Shortened Timelines
Traditional antibody discovery requires 3–6 months.
Off-the-shelf sequence transfer can provide same-day delivery—crucial for time-sensitive programs.
2) Significant Cost Reduction
No need for immunization, screening, or sequence analysis.
Resources go directly into engineering and functional validation.
3) Lower Early-Stage Risk
DIMA BIOTECH sequences originate from mature discovery platforms and undergo expression, affinity, specificity, or functional assessments—reducing early failure rates.
4) Clear and Safe IP Pathways
Sequence transfer ensures full commercial-use rights and minimizes future IP disputes.
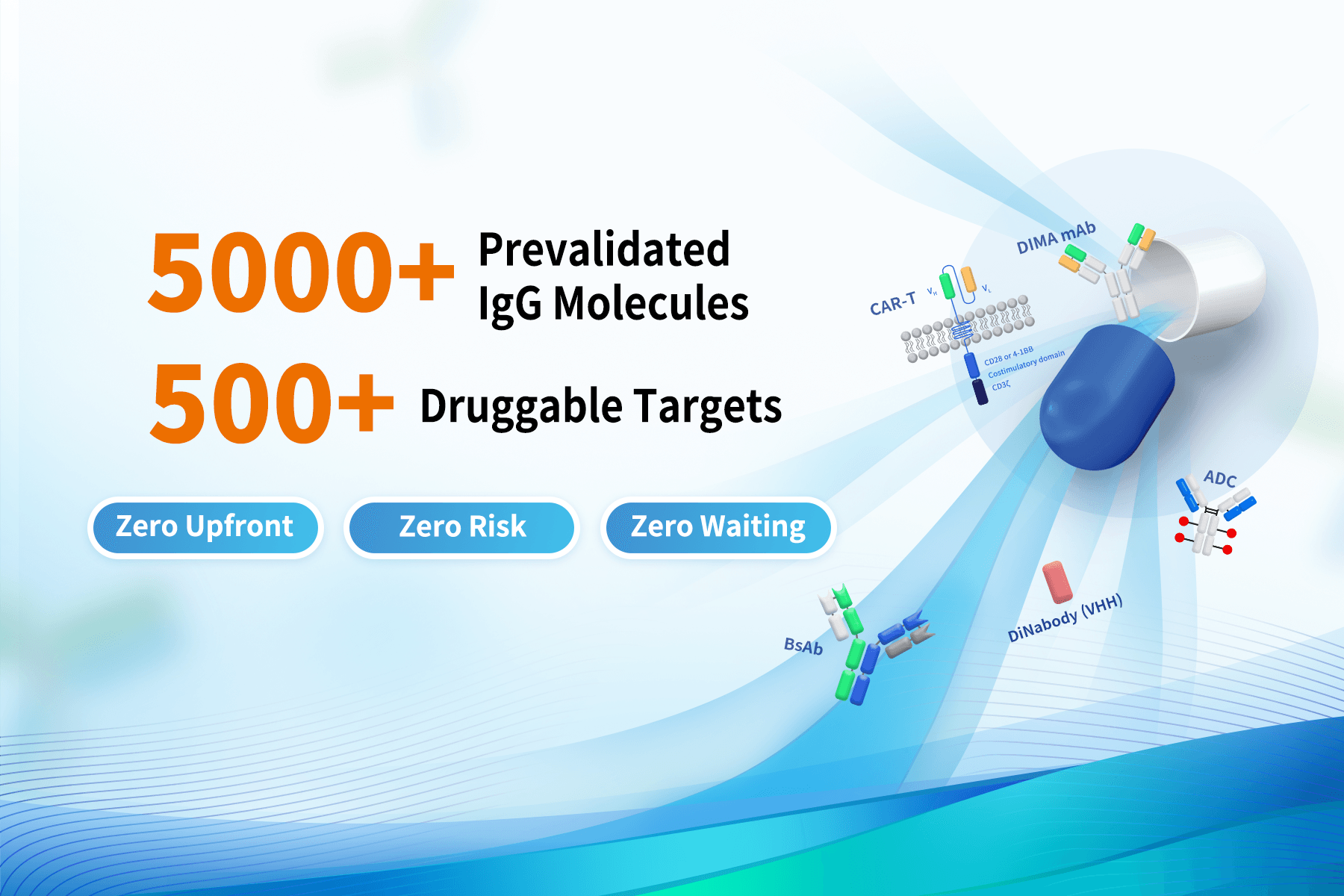
3.DIMA BIOTECH: A True “Off-the-Shelf + Zero-Cycle, Zero-Down-Payment, Zero-Risk” Antibody Acceleration Platform
- Real Off-the-Shelf Delivery — Zero Cycle
Not custom. No waiting. All lead sequences are cloned, validated, documented, and ready for same-day transfer, covering oncology, autoimmunity, inflammation, and neurology targets with clear IP status.
- Evaluate First, License Later — Zero Upfront
Access the full development data package before payment.
Only move to licensing after satisfactory evaluation—ideal for biotechs managing multiple targets with limited budgets.
- Full Verification System — Zero Risk
Each sequence undergoes recombinant antibody–level validation, including: specificity, affinity (SPR/BLI), functional activity combined with DIMA BIOTECH’s extensive engineering experience, this ensures delivery of true lead antibodies—not basic research-grade molecules.
- B-Cell Expansion Platform for Higher-Quality Leads
Generates hundreds of microliters of supernatant for functional screening and delivers functional candidates in ~10 days.
- Antibodies + Proteins in Stock for Fast Functional Readouts
ECD proteins, multi-membrane proteins, and multi-epitope antigens are available as off-the-shelf reagents, enabling seamless sequence-to-function workflows.
- End-to-End Acceleration From Sequence to IND
Covers expression, purification, humanization, affinity maturation, and in-vitro validation within one integrated platform.
- Broad Industry Validation
500+ targets, 800+ partners, and multiple molecules already in preclinical or clinical stages.
4.Licensing Options: Flexible for All Program Stages

Figure 1. The Licensing Options of DIMA BIOTECH’s IgG Sequences.
5. How to Access DIMA’s Off-the-Shelf Antibody Sequences
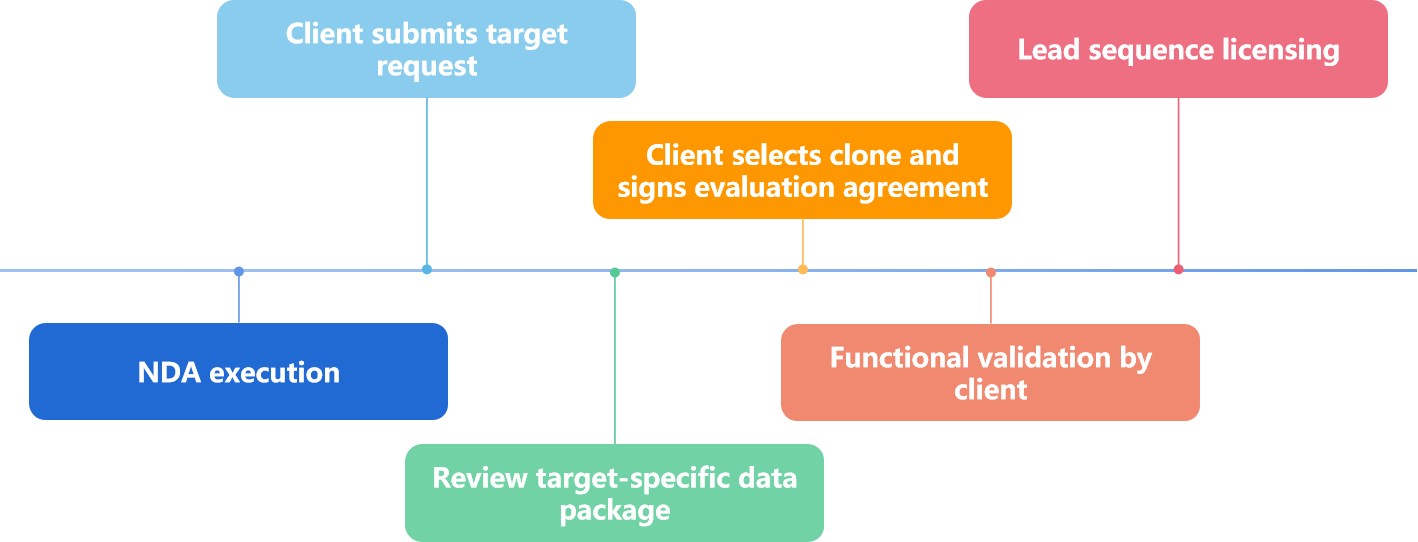
Figure 2. The Process of Access DIMA BIOTECH’s Off-the-Shelf Antibody Sequences.
Conclusion: Turning Antibody Discovery From “Delay” to “Acceleration”
Antibody R&D should no longer be slowed by prolonged screening cycles. As the industry moves toward faster, more predictable development models, immediately accessible, IP-clear, fully validated antibody sequences will become a key competitive advantage.
With off-the-shelf lead sequences + a full-stack antibody development platform, DIMA BIOTECH helps teams stay ahead of timelines, shorten development cycles, and improve success rates.
If you are looking for high-quality, exclusive, same-day-deliverable antibody sequences, DIMA BIOTECH is your most reliable partner.