In the era of continuous innovation in immuno-oncology treatment, the news of the approval by the China National Medical Products Administration (NMPA) for the clinical trial of the innovative anti-PD-L1/VEGF bispecific antibody HLX37 from Henlius Biotech has once again brought the PD-1/PD-L1 immune checkpoint-targeted pathway into the spotlight of industry attention. As a dual-target strategy connecting immune regulation and tumor microenvironment angiogenesis, the PD-L1/VEGF bispecific antibody not only continues the successful approach of PD-1/PD-L1 immune suppression blockade but also explores a new mechanism of synergy between immunotherapy and anti-angiogenesis. This advancement represents the increasing influence of domestic innovative biologics in the global immuno-oncology field and reflects the importance of a deeper understanding of the structure, expression regulation, and signaling mechanisms of the PD-1/PD-L1 pathway for novel drug design and precision treatment strategies, especially as monoclonal antibody efficacy faces challenges of resistance and differences in response rates.
1.Overview of the PD-1 and PD-L1 Targets
Programmed Cell Death Protein 1 (PD-1, gene name PDCD1) is an important immune inhibitory receptor primarily expressed on the surface of activated T cells, but it can also be detected on B cells, natural killer (NK) cells, and some dendritic cells. PD-1 was first discovered in 1992 and was initially thought to be related to apoptosis. Subsequent research gradually clarified its key role in immune negative regulation [1].
The main ligands of PD-1 include PD-L1 (Programmed Death-Ligand 1, also known as B7-H1, gene name CD274) and PD-L2 (B7-DC). Among these, PD-L1 has the most prominent research and clinical significance. PD-L1 is expressed not only on antigen-presenting cells (such as dendritic cells, macrophages, and B cells) but also induced on various non-immune cells and tumor cells. Tumor cells high in PD-L1 expression can bind to PD-1 on T cells, thereby suppressing anti-tumor immune responses, forming the classic “immune evasion” mechanism [2].
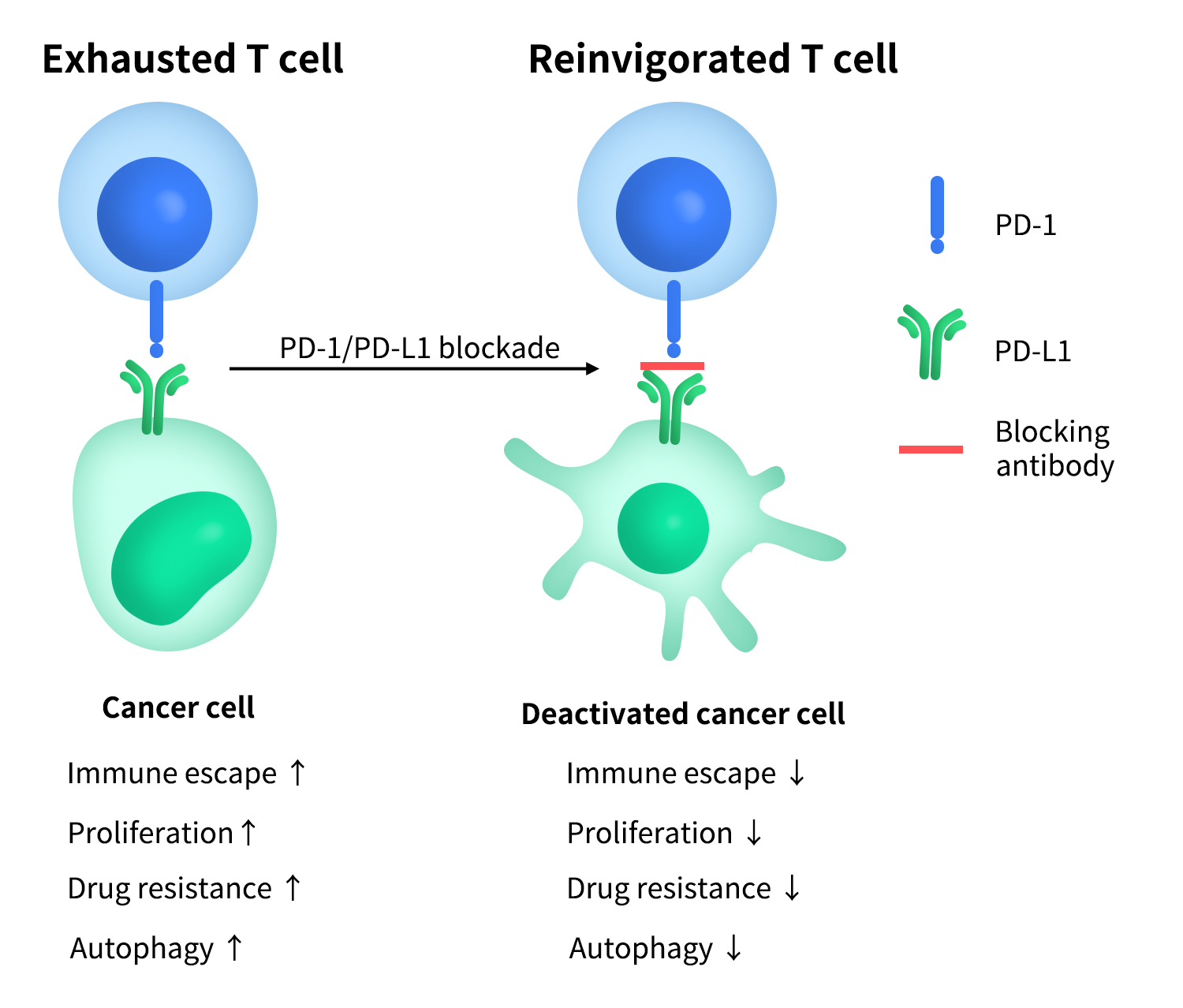
Figure 1.PD-1 & PD-L1 ‘immune evasion’ mechanism
Based on this biological foundation, the PD-1/PD-L1 pathway has become one of the most important and mature immune checkpoint targets in current tumor immunotherapy, with related monoclonal antibodies achieving breakthrough efficacy in multiple tumor indications.
2.Structural Features of PD-1 and PD-L1
PD-1 is a type I transmembrane protein, and its overall structure can be divided into three functional regions: the extracellular region consists of a V-set immunoglobulin-like domain, which is the key region for the specific binding of PD-1 to PD-L1/PD-L2; the transmembrane region is a single α-helix structure that connects the extracellular ligand-binding event to intracellular signal transduction; although the intracellular region is short, it is highly functional, containing two critical tyrosine motifs, ITIM (Immunoreceptor Tyrosine-based Inhibitory Motif) and ITSM (Immunoreceptor Tyrosine-based Switch Motif). Among these, ITSM is considered the core functional site that mediates PD-1 inhibitory signaling. Unlike classical activating immune receptors, PD-1 does not possess intrinsic kinase activity, and its immune inhibitory function entirely depends on the ability of its intracellular motifs to recruit downstream signaling molecules, such as SHP-2, after receptor activation.
2.2 Molecular Structure of PD-L1
PD-L1 is also a type I transmembrane protein, with its extracellular region consisting of two immunoglobulin-like domains (IgV+IgC). The IgV domain not only directly participates in the binding with PD-1 but also mediates its interaction with B7-1 (CD80). Several crystallographic and cryo-electron microscopy studies have resolved the high-resolution structure of the human PD-1/PD-L1 complex, systematically elucidating the binding interface, spatial conformational features, and key amino acid residues, which provide important structural insights for understanding the dual roles of PD-L1 in immune suppression and immune regulation [3, 4].
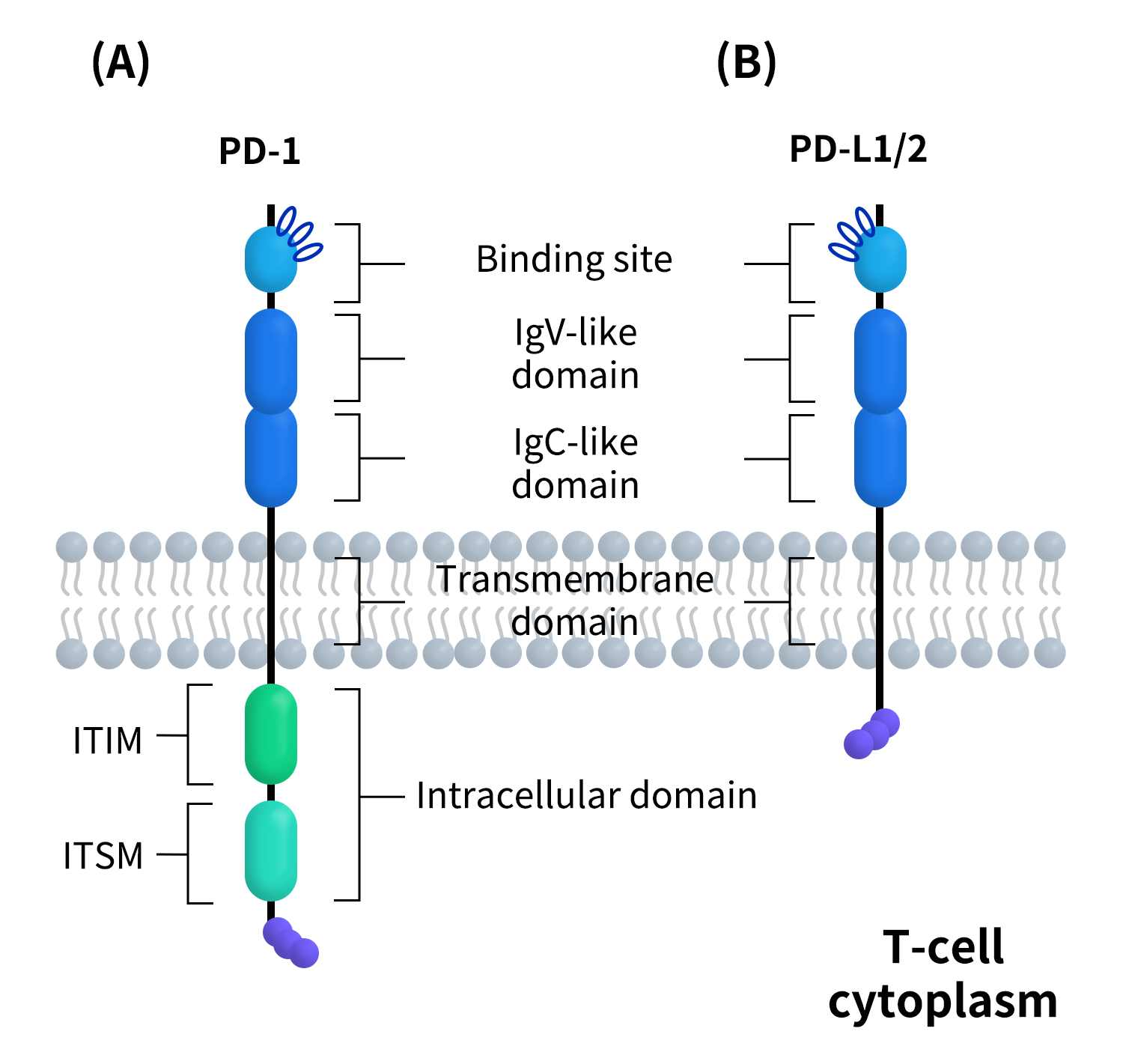
Figure 2.The structure of PD-1&PD-L1
3.Tissue Distribution and Expression of PD-1/PD-L1
Under normal physiological conditions, the expression of the PD-1/PD-L1 pathway shows distinct cell type and tissue specificity. Its distribution is closely related to maintaining peripheral immune tolerance and preventing excessive immune responses. However, in tumors and chronic inflammation, this pathway is often aberrantly activated and significantly upregulated, becoming a critical molecular basis for immune evasion.
3.1 Expression Characteristics of PD-1
Under steady-state conditions, PD-1 expression is low or even undetectable in naive T cells. When T cells are stimulated by antigens and activated, PD-1 expression is rapidly upregulated as a negative feedback mechanism to limit excessive immune responses. After the clearance of acute infections, PD-1 expression gradually decreases as the immune response subsides.
However, in the context of tumors or chronic infections (e.g., persistent viral infections), where antigen stimulation persists, PD-1 is often continuously expressed at high levels, becoming an important molecular marker of T cell exhaustion.
3.2 Induced Expression of PD-L1
In contrast to PD-1, the regulation of PD-L1 expression is more complex. Inflammatory factors, especially IFN-γ, are considered the most important upstream signals for inducing PD-L1 expression. In the tumor microenvironment, tumor cells, tumor-associated macrophages, and fibroblasts can significantly upregulate PD-L1 in response to stimuli such as IFN-γ and TNF-α, thereby creating a strong inhibitory state for local immune responses [2].
In addition, the expression of PD-L1 is also regulated by various non-inflammatory factors, including gene amplification, activation of transcription factors (such as STAT3, HIF-1α), and epigenetic modifications. This explains why the high expression of PD-L1 in some tumors does not entirely rely on the immune-inflammatory context.
4.Mechanisms of the PD-1/PD-L1 Signaling Pathway
The core function of the PD-1/PD-L1 pathway is to act as a “brake” on T cell receptor (TCR)-mediated activation signals. Its signaling mechanism primarily relies on the ITSM motif within the intracellular region of PD-1.
4.1 Signal Initiation: Ligand Binding and Tyrosine Phosphorylation
When PD-1 binds to PD-L1 or PD-L2, the ITSM motif in the intracellular tail of PD-1 undergoes phosphorylation. This phosphorylation event provides binding sites for downstream proteins containing SH2 domains and marks the starting point for inhibitory signal transduction [5].
4.2 Recruitment of SHP-2 and Inhibition of Key Signaling Molecules
The phosphorylated ITSM motif mainly recruits the protein tyrosine phosphatase SHP-2 (SHP-1 is also involved in some contexts). SHP-2 exerts its inhibitory effects by dephosphorylating multiple signaling pathways closely related to T cell activation, including:
- TCR-CD3-ZAP70 pathway:Reduces the strength of TCR signaling;
- CD28 co-stimulation pathway: Inhibiting co-stimulatory signals is a key mechanism of PD-1’s inhibitory effect;
- PI3K–AKT–mTOR pathway:Affects T cell metabolism, survival, and proliferation;
- RAS–MEK–ERK pathway:Inhibits the activation of the transcription factor AP-1.
The combined suppression of these signals results in reduced secretion of cytokines such as IL-2, decreased proliferation, and limited effector functions of T cells [5,6].
4.3 T Cell Exhaustion and Metabolic Reprogramming
Under conditions of persistent antigen stimulation, such as in chronic infections or tumors, prolonged high expression and activation of PD-1 drive T cells into an “exhausted” state, characterized by reduced cytotoxicity, diminished cytokine production, and impaired metabolic capacity. Studies have shown that PD-1 signaling significantly inhibits glycolysis and impacts mitochondrial function, thereby limiting T cell sustained effector activity at the metabolic level [7].
4.4 Synergy with Other Immune Checkpoints
It is important to note that PD-1/PD-L1 does not act in isolation. Its signaling pathway intersects and collaborates with other immune checkpoints, such as CTLA-4, LAG-3, and TIM-3, which provides an important theoretical basis for the development of combination immunotherapy strategies [2]. Regarding the differences between targeting PD-1 and PD-L1…
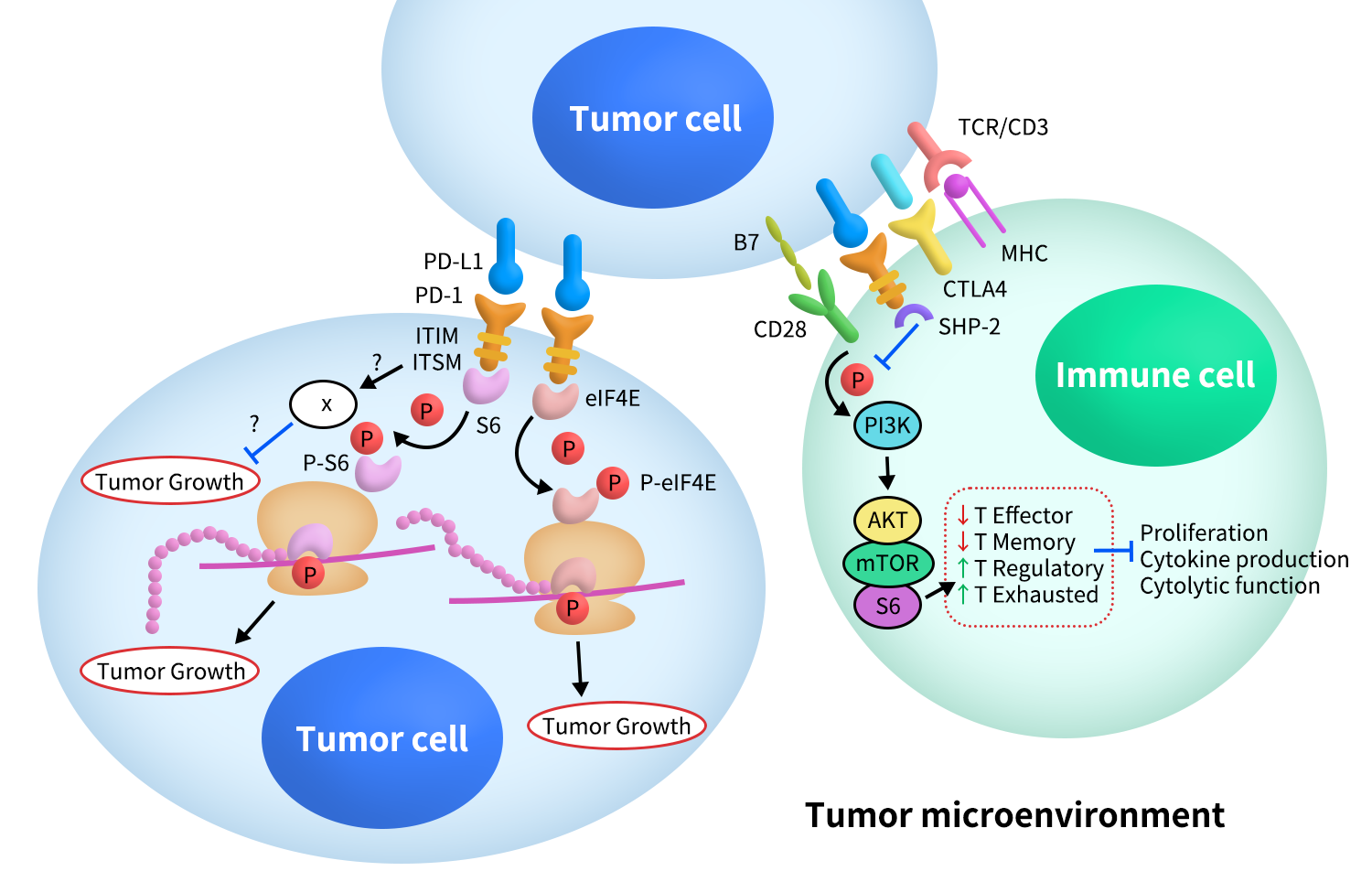
Figure 3.The Signaling of PD-1&PD-L1
5.Diseases Associated with PD-1/PD-L1
The PD-1 and PD-L1 pathway is a part of immune system regulation, playing a key role in maintaining immune tolerance and immune evasion. By interacting with each other, PD-1 and PD-L1 inhibit T cell functions, thereby preventing excessive immune responses. While this mechanism is essential in preventing autoimmune diseases and overactive immune reactions, it is also exploited by tumor cells to evade immune surveillance. Consequently, dysregulation of the PD-1/PD-L1 pathway is closely associated with the development of various diseases, including cancer, autoimmune diseases, and transplant rejection.
5.1 Cancer: A Major Mechanism of Immune Evasion
Cancer cells exploit the PD-1/PD-L1 signaling pathway to evade immune system surveillance. Under normal conditions, T cells recognize abnormal antigens on the surface or inside of cancer cells and mediate immune killing. However, many tumor cells upregulate PD-L1 expression, blocking T cell function and preventing immune system clearance. By binding to PD-1 on T cells, tumor cells suppress T cell activation and proliferation, allowing them to escape immune surveillance and attack. Tumor types related to this mechanism include:
Non-Small Cell Lung Cancer (NSCLC): NSCLC was one of the first cancers to benefit from immune checkpoint inhibitors. Studies have shown that PD-L1 expression is closely related to immune evasion in NSCLC, and anti-PD-1 antibodies (such as nivolumab) significantly improve survival in these patients.
Melanoma: Melanoma patients often exhibit high PD-L1 expression. As a result, anti-PD-1 treatments like pembrolizumab (Keytruda) and nivolumab have become significant treatment options for melanoma.
Head and Neck Squamous Cell Carcinoma: PD-1/PD-L1 inhibitors have become important treatment choices for head and neck squamous cell carcinoma, particularly for patients with high PD-L1 expression.
Bladder Cancer and Renal Cell Carcinoma: PD-1/PD-L1 inhibitors have also shown promising efficacy in bladder cancer and renal cell carcinoma, especially in patients who have failed traditional therapies.
Immune checkpoint inhibitors work by removing the inhibitory effect of PD-1/PD-L1, thereby restoring T cell anti-tumor activity and becoming a breakthrough method for treating these cancers.
5.2 Immune-Related Diseases: Dysregulation of PD-1/PD-L1 and Autoimmune Reactions
The PD-1/PD-L1 pathway not only plays a critical role in tumor immune evasion but is also closely related to the development of various autoimmune diseases. Autoimmune diseases are often caused by the immune system attacking the body’s own tissues, leading to inflammation and damage. Dysregulation of the PD-1/PD-L1 pathway can cause an overactive immune system, triggering these diseases. Related diseases include:
- Systemic Lupus Erythematosus (SLE): SLE is a typical autoimmune disease in which the immune system attacks normal tissues. Studies have shown that PD-1 expression is often reduced in SLE patients, and dysfunction of PD-L1 may promote immune cells attacking normal tissues. In some SLE patients, the lack of PD-1 or inhibition of its signaling pathway may be related to disease activity and worsening symptoms.
- Rheumatoid Arthritis: In rheumatoid arthritis patients, T cells attack joint tissues, causing chronic inflammation. Dysfunction of PD-1 may be related to abnormal T cell activation in the joints. Therefore, regulation of PD-1/PD-L1 may become a potential target for treating rheumatoid arthritis.
- Multiple Sclerosis (MS): MS is an autoimmune disease of the central nervous system, and the PD-1/PD-L1 pathway plays an important role in the pathogenesis of MS. Studies have found that PD-1 is dysfunctional in MS patients, potentially leading to a disorder in autoimmune responses, resulting in damage to the nervous system.
- Type 1 Diabetes: Type 1 diabetes is caused by the immune system attacking pancreatic β Dysregulation of PD-1/PD-L1 signaling is thought to be related to the onset of this disease. In certain animal models, restoring PD-1 function has been shown to reduce β cell destruction and slow disease progression.
5.3 Role of PD-1/PD-L1 in Organ Transplantation
In organ transplantation, the PD-1/PD-L1 signaling pathway also plays a crucial role. During transplantation, the recipient’s immune system may recognize the transplant as foreign and initiate an immune response leading to rejection. The PD-1/PD-L1 signaling pathway helps the immune system recognize and adapt to these foreign tissues, preventing rejection. Studies have shown that PD-L1 plays a key role in regulating transplant immune tolerance. In some transplant patients, modulating the PD-1/PD-L1 pathway can effectively suppress rejection and promote long-term graft survival.
As immune checkpoint molecules, PD-1/PD-L1 have become central targets in cancer immunotherapy, achieving significant clinical results. By blocking the interaction between PD-1 and PD-L1, immune suppression on T cells can be effectively lifted, restoring their anti-tumor immune function. Immune checkpoint inhibitors based on this mechanism have been widely used in cancers such as melanoma, non-small cell lung cancer, renal cell carcinoma, and Hodgkin lymphoma, significantly improving the long-term survival of some patients [8].
At the same time, resistance mechanisms, predictive biomarkers for efficacy, and immune-related adverse events (irAEs) remain key research areas in this field. Further investigation into these issues relies heavily on high-quality PD-1/PD-L1 proteins and antibody reagents.
Explore more information of the latest clinical progress of drugs targeting PD-1
Explore more information of the latest clinical progress of drugs targeting PD-L1
6.DIMA PD-1/PD-L1-Related Protein and Antibody Product Support
DIMA offers a range of recombinant proteins and monoclonal antibodies targeting the PD-1/PD-L1 pathway, covering multiple species such as human, mouse, and canine. These products are widely used in immune checkpoint research, immunotherapy development, antibody screening, drug screening, and mechanism studies, helping researchers explore the function of the PD-1/PD-L1 pathway and its role in cancer immunotherapy. Our high-quality products provide reliable experimental support, contributing to the advancement of research in related fields.
- PD-1/PD-L1 Target-Related Products
|
Target |
Product_Type |
SKU |
Product name |
|
PD-1 |
ECD Proteins |
PME100025 |
|
|
|
|
PME100461 |
|
|
|
|
PME100462 |
|
|
|
|
PME-M100099 |
|
|
|
|
PME-D100005 |
|
|
|
|
PME-D100006 |
|
|
|
|
PME-D100008 |
|
|
|
|
PME101789 |
|
|
|
Monoclonal antibodies |
DME100177 |
|
|
|
|
DME100177B |
|
|
|
|
DME101041 |
|
|
|
|
DME101041B |
|
|
PD-L1 |
ECD Proteins |
PME100023 |
|
|
|
|
PME100480 |
|
|
|
|
PME-M100077 |
|
|
|
|
PME-C100021 |
|
|
|
|
PME-D100007 |
|
|
|
|
PME-D100009 |
|
|
|
|
PME101591 |
|
|
|
|
PME101592 |
|
|
|
|
PME101706 |
|
|
|
Monoclonal antibodies |
DME100123 |
|
|
|
|
DME100124 |
|
|
|
|
DMC100875 |
|
|
|
|
DME100123B |
|
|
|
|
DME100124B |
|
|
|
|
DMC100875B |
|
|
PD-L2 |
ECD Proteins |
PME100518 |
|
|
|
Monoclonal antibodies |
DME100169 |
|
|
|
|
DME100169B |
|
|
|
|
DME100169P |
|
|
B7-1/CD80 |
ECD Proteins |
PME100473 |
|
|
|
|
PME100047 |
|
|
|
|
PME-M100044 |
|
|
|
Monoclonal antibodies |
DME100109 |
|
|
|
|
DME100110 |
|
|
|
|
DME100111 |
|
|
|
|
DME100109B |
|
|
|
|
DME100110B |
|
|
|
|
DME100111B |
|
|
|
|
DME100109P |
- Progress of PD-1/PD-L1 Lead Antibodies

References
- Ishida Y, Agata Y, Shibahara K, Honjo T. Identification of PD-1 as a novel immunoregulatory receptor. Journal of Experimental Medicine. 1992.
- Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015.
- Zak KM, Kitel R, Przetocka S, et al. Structure of the complex of human PD-1 and PD-L1. Structure. 2015.
- Lin DY, Tanaka Y, Iwasaki M, et al. The PD-1/PD-L1 complex resembles the antigen-binding Fv domains of antibodies. Proceedings of the National Academy of Sciences USA. 2008.
- Okazaki T, Honjo T. PD-1 and PD-1 ligands: from discovery to clinical application. International Immunology. 2007.
- Strazza M, Azoulay-Alfaguter I, Peled M, et al. SHP-2 suppresses T cell activation downstream of PD-1. Nature Immunology. 2021.
- Patsoukis N, Bardhan K, Chatterjee P, et al. PD-1 alters T-cell metabolic reprogramming. Nature Immunology. 2015.
- Han Y, Liu D, Li L. PD-1/PD-L1 pathway: current researches in cancer. American Journal of Cancer Research. 2020.
