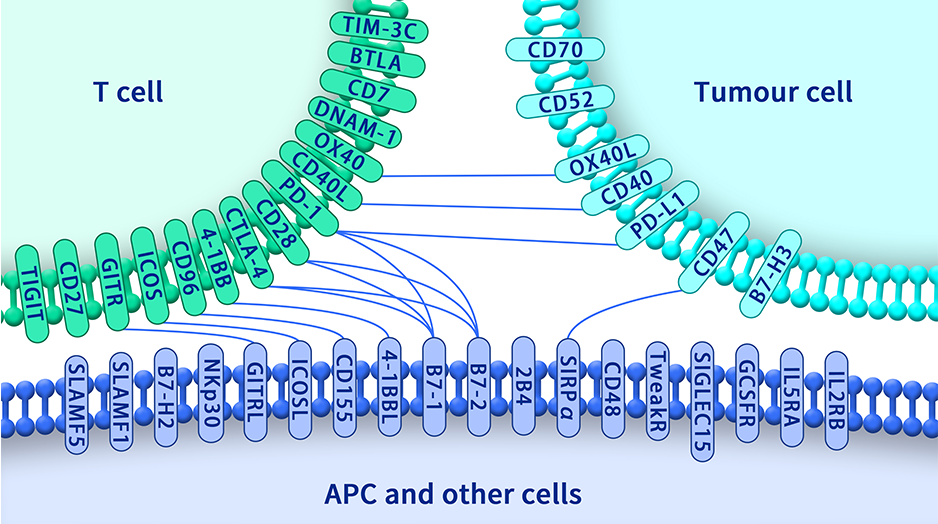
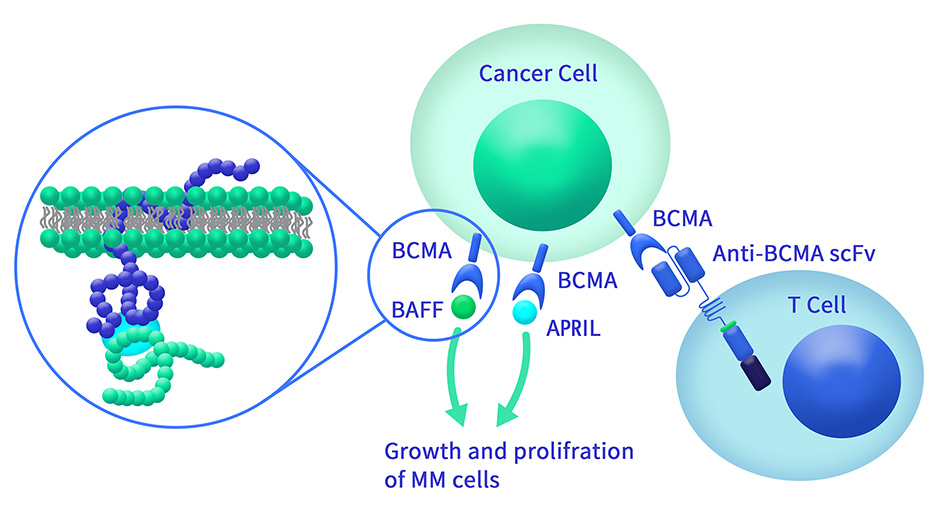
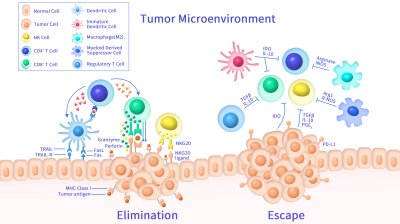
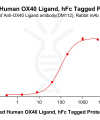
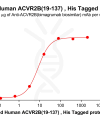
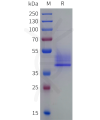
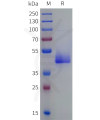
Extracellular domain proteins, also abbrevated as ECD proteins, are parts of membrane proteins located outside the cell membrane, interacting with the environment. They are crucial for signal recognition, ligand binding, and intercellular communication. Many receptor drug targets, like GPCRs, RTKs, and immune checkpoints (e.g., PD-1, CTLA-4), rely on their extracellular domains for biological activity. ECDs play roles in immune responses, tumorigenesis, and viral infections. Due to the difficulty in stabilizing membrane proteins, ECDs are essential for membrane protein research and drug screening. By removing transmembrane regions, soluble, well-folded recombinant ECDs are produced, suitable for assays like ELISA, SPR, and antibody screening.
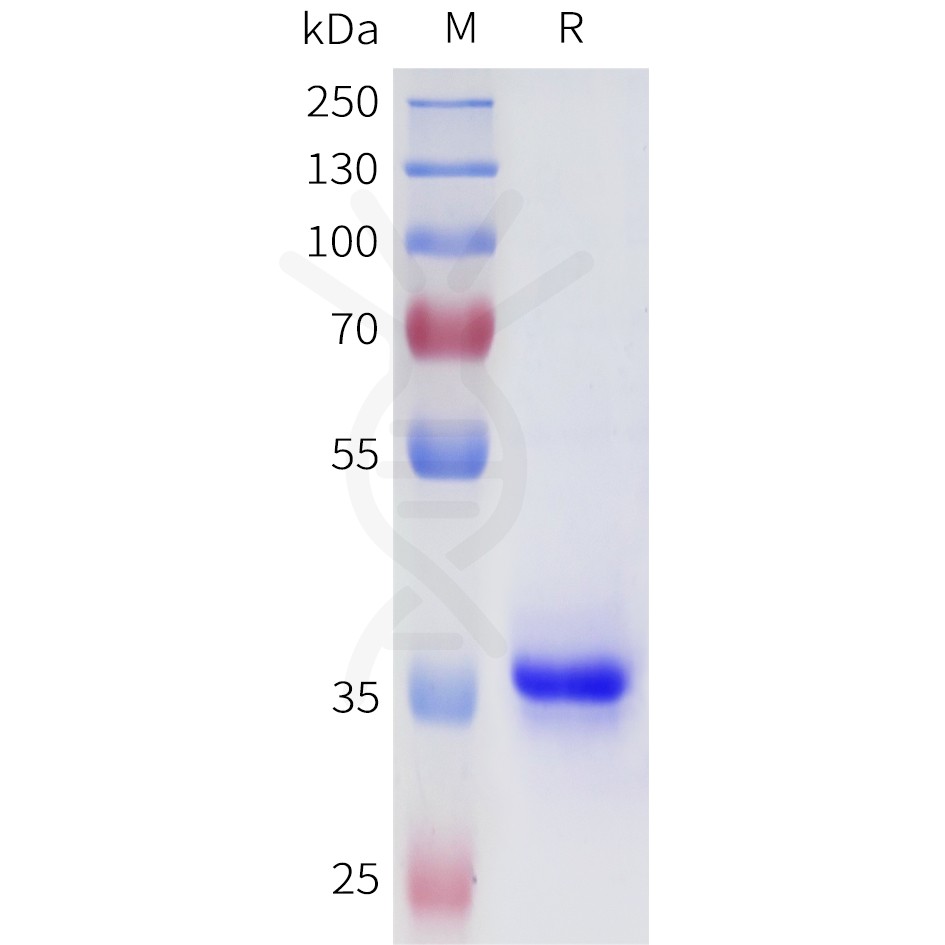
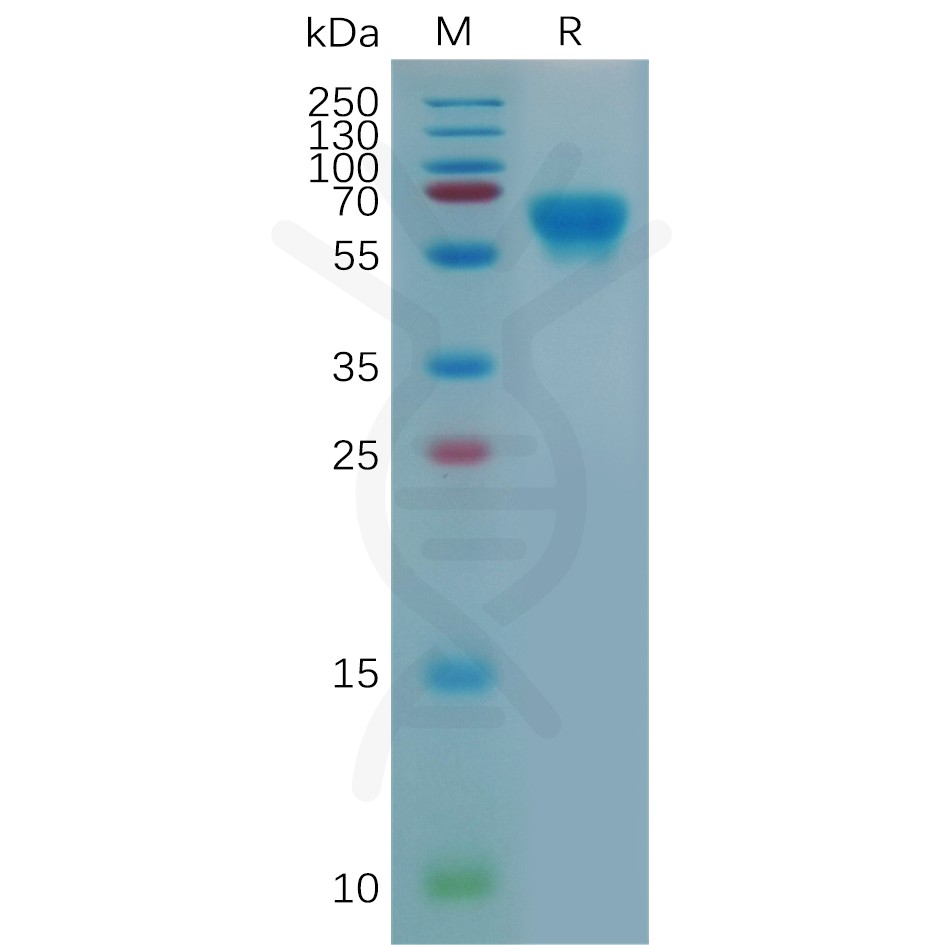
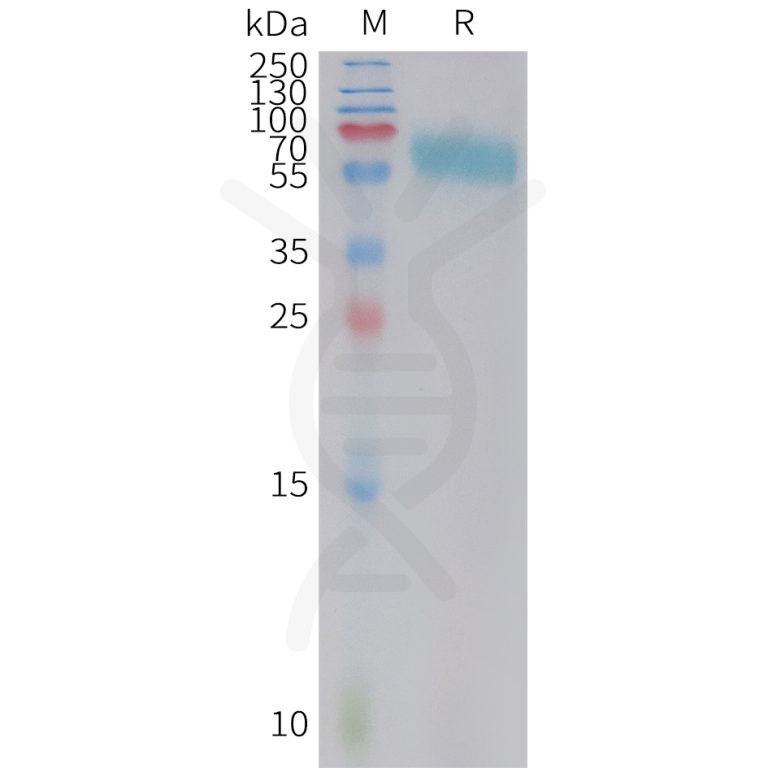
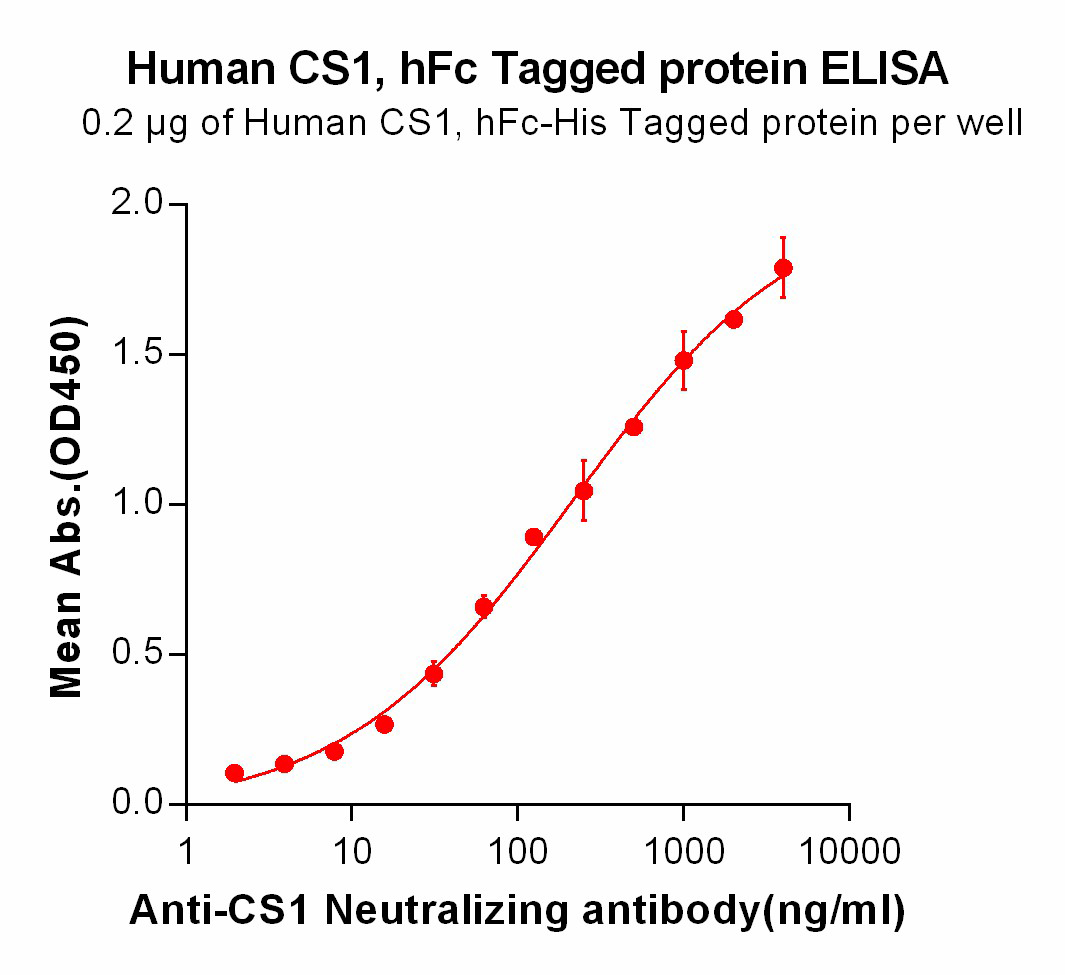
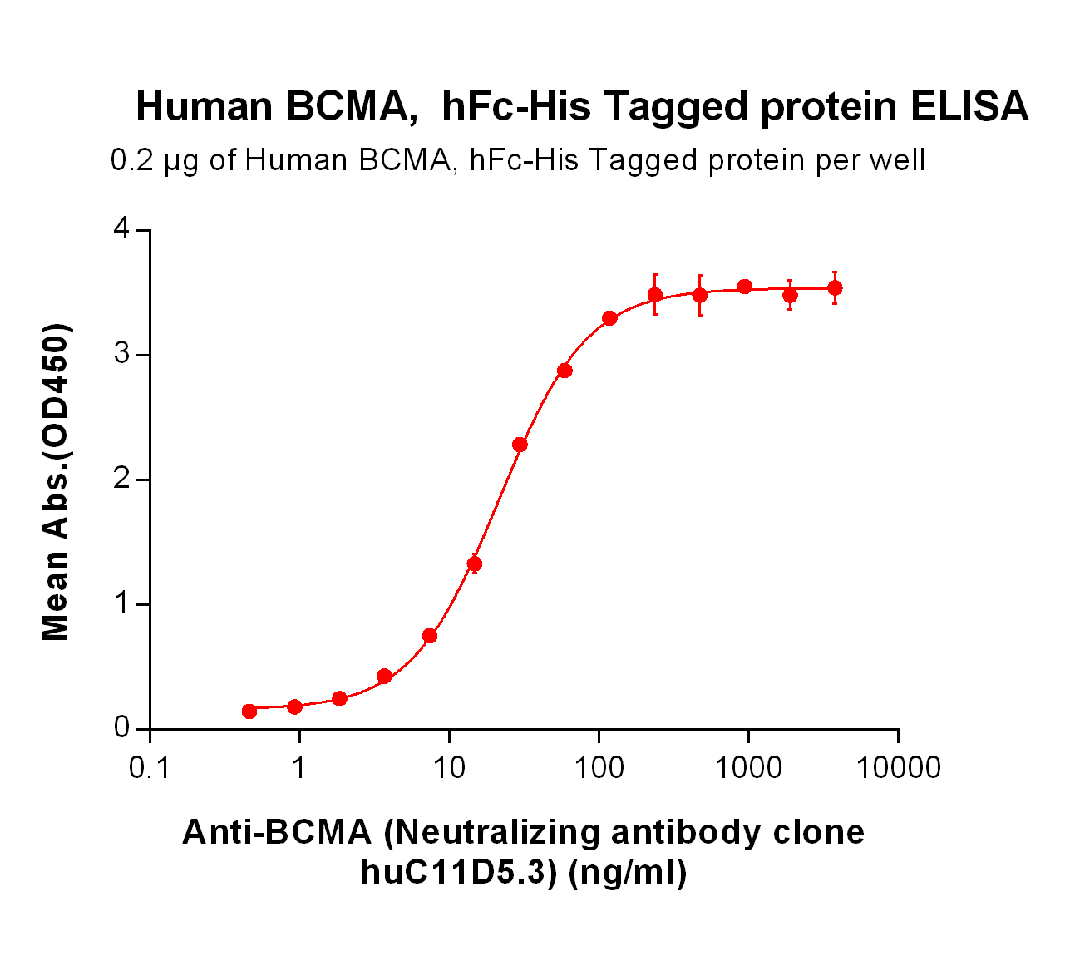
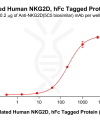
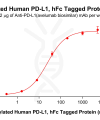
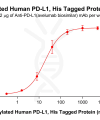
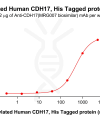
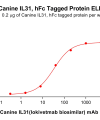
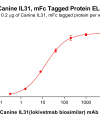
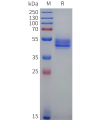
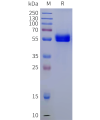
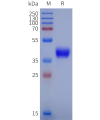
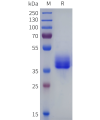
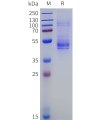
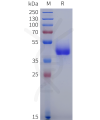
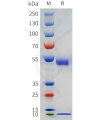
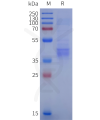
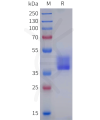
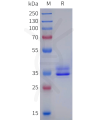
DIMA BIOTECH’s ECD proteins, primarily targeting single-pass membrane proteins, have successfully expressed over 1600 high-purity ECDs, covering over 900 targets, including GPCRs, immune checkpoints, tumor antigens, and metabolic receptors. More than 500 of these have corresponding flow cytometry antibodies. All ECD proteins are validated for purity via SDS-PAGE and activity via ELISA, supporting antibody screening, functional verification, and structural studies for membrane protein drug development.