Immune checkpoints
1. Overview of Immune Checkpoints
Immune checkpoints are critical regulatory pathways that control the activation, intensity, and duration of immune responses. They are primarily mediated through interactions between receptors and ligands expressed on immune cells and their counterparts in the tissue microenvironment. Under physiological conditions, immune checkpoints maintain immune homeostasis and prevent excessive immune activation.
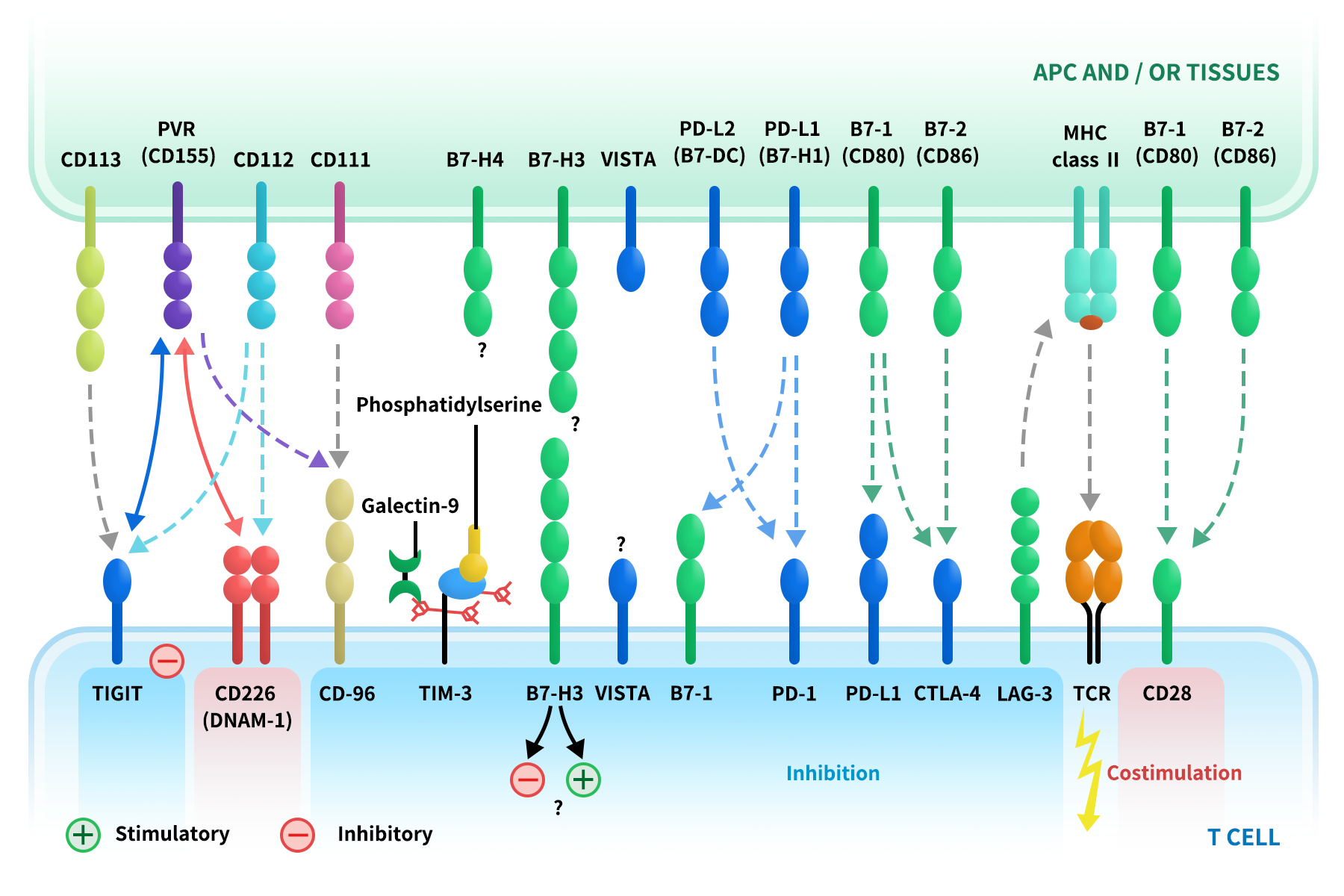
The figure of immune checkpoints and their receptors
In cancer and other chronic diseases, tumor cells often exploit immune checkpoint pathways by overexpressing inhibitory ligands such as PD-L1 or CD47, thereby suppressing antitumor immune responses. As a result, immune checkpoint blockade has become one of the most successful therapeutic strategies in modern immuno-oncology.
From a drug discovery perspective, most immune checkpoint molecules are transmembrane proteins, and their biological activity is largely determined by interactions mediated through the extracellular domain (ECD). High-quality ECD proteins with correct folding and native-like conformation are therefore essential tools for antibody discovery, affinity maturation, epitope mapping, and functional characterization.
Common Features of Immune Checkpoints
- Predominantly type I or type II transmembrane proteins
- Functional interactions mediated by extracellular domains
- Well-defined mechanisms with clear translational relevance
- Strong and growing clinical validation
Application of DIMA BIOTECH's Products
- Antibody discovery and primary screening
- Conformational and epitope characterization
- Mechanistic and functional studies
- Assay development and reference standards
2. Key Immune Checkpoint Targets
PD-1/PD-L1 Immune Checkpoint Axis
The PD-1/PD-L1 axis is the most clinically validated immune checkpoint pathway, mediating T cell inhibition through receptor–ligand interactions and serving as a core target for antibody discovery and immuno-oncology drug development. Explore PD-1/PD-L1 Pathway Blog…
LAG-3/MHC Class II Immune Checkpoint Axis
The LAG-3/MHC II axis contributes to T cell exhaustion and immune suppression, often acting synergistically with PD-1, and is an important target for dual-pathway and combination immune checkpoint strategies.
Besides these, additional immune checkpoints such as TIM-3, VISTA, B7-H3, B7-H4, and CD47/SIRPα continue to show strong therapeutic relevance and expanding potential across oncology and immune-related disease research. DIMA BIOTECH has developed a series of high-quality recombinant proteins on immune checkpoint targets . All these proteins are manufactured in HEK293 cells. It is very likely that these recombinant proteins will maintain three-dimensional structure close to native proteins and the authentic post-translational modifications specific to human cells.
3. DIMA BIOTEH's Product Features
Ligand-receptor interaction evaluation:
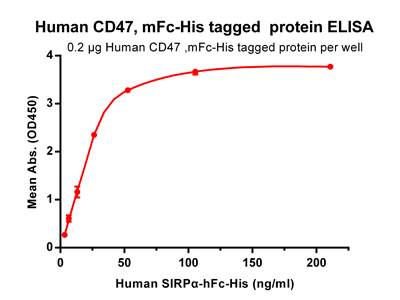
Figure 2. ELISA plate pre-coated by 2 ug/ml (100 ul/well) Human CD47, mFc-His tagged protein (PME100008) can bind its native ligand Human SIRPα, hFc-His tagged protein (PME100009) in a linear range of 3.3-26.37 ng/ml.
Biosimilar antibody interaction evaluation
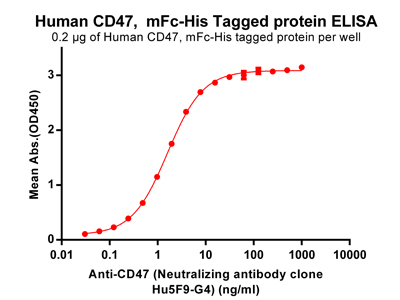
Figure 3. ELISA plate pre-coated by 2 ug/ml (100 ul/well) Human CD47, mFc-His tagged protein (PME100008) can bind Anti-CD47 (Neutralizing antibody clone Hu5F9-G4)(BME100001) in a linear range of 0.061-1.606 ng/ml.
Rigorous quality testing
Every batch of recombinant proteins are under rigorous quality testing to make sure the protein stability.
Stability test 1: Ligand-receptor interaction testing after multiple freeze-thaw cycles
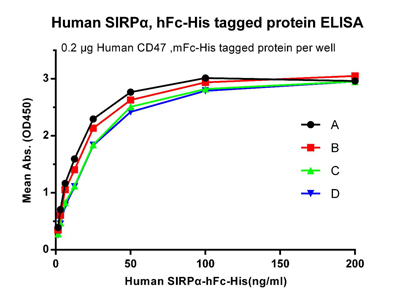
Figure 4. Human SIRPα, hFc-His tagged protein without freeze-thaw treatment. B: Human SIRPα, hFc-His tagged protein after one freeze-thaw cycle. C: Human SIRPα, hFc-His tagged protein after three freeze-thaw cycles. D: Human SIRPα, hFc-His tagged protein after five freeze-thaw cycles.
Stability test2: Protein integrity analysis after multiple freeze-thaw cycles
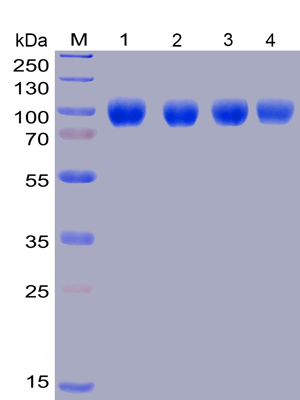
Figure 5. Lane 1: Human SIRPα, hFc-His tagged protein without freeze-thaw treatment, Lane 2: Human SIRPα, hFc-His tagged protein after one freeze-thaw cycle, Lane 3: Human SIRPα, hFc-His tagged protein after three freeze-thaw cycles, Lane 4: Human SIRPα, hFc-His tagged protein after five freeze-thaw.
